当前位置:
X-MOL 学术
›
J. Mol. Recognit.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Understanding the disrupting mechanism of the Tau aggregation motif "306 VQIVYK311 " by phenylthiazolyl-hydrazides inhibitors.
Journal of Molecular Recognition ( IF 2.7 ) Pub Date : 2020-03-30 , DOI: 10.1002/jmr.2848 Elena Moreno-Castillo 1 , Yoanna M Álvarez-Ginarte 1 , Mario E Valdés-Tresanco 2 , Luis A Montero-Cabrera 1 , Ernesto Moreno 3 , Pedro A Valiente 2
Journal of Molecular Recognition ( IF 2.7 ) Pub Date : 2020-03-30 , DOI: 10.1002/jmr.2848 Elena Moreno-Castillo 1 , Yoanna M Álvarez-Ginarte 1 , Mario E Valdés-Tresanco 2 , Luis A Montero-Cabrera 1 , Ernesto Moreno 3 , Pedro A Valiente 2
Affiliation
|
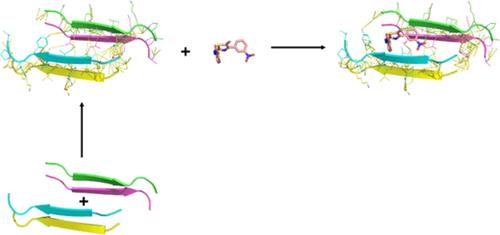
Alzheimer's disease is a progressive neurodegenerative disorder characterized by the abnormal processing of the Tau and the amyloid precursor proteins. The unusual aggregation of Tau is based on the formation of intermolecular β‐sheets through two motifs: 275VQIINK280 and 306VQIVYK311. Phenylthiazolyl‐hydrazides (PTHs) are capable of inhibiting/disassembling Tau aggregates. However, the disaggregation mechanism of Tau oligomers by PTHs is still unknown. In this work, we studied the disruption of the oligomeric form of the Tau motif 306VQIVYK311 by PTHs through molecular docking, molecular dynamics, and free energy calculations. We predicted hydrophobic interactions as the major driving forces for the stabilization of Tau oligomer, with V306 and I308 being the major contributors. Nonpolar component of the binding free energy is essential to stabilize Tau‐PTH complexes. PTHs disrupted mainly the van der Waals interactions between the monomers, leading to oligomer destabilization. Destabilization of full Tau filament by PTHs and emodin was not observed in the sampled 20 ns; however, in all cases, the nonpolar component of the binding free energy is essential for the formation of Tau filament‐PTH and Tau filament‐emodin. These results provide useful clues for the design of more effective Tau‐aggregation inhibitors.
中文翻译:

了解苯基噻唑基酰肼抑制剂对 Tau 聚集基序“306 VQIVYK311”的破坏机制。
阿尔茨海默病是一种进行性神经退行性疾病,其特征在于 Tau 和淀粉样前体蛋白的异常加工。Tau 的异常聚集基于通过两个基序形成分子间 β-折叠:275 VQIINK 280和306 VQIVYK 311。苯基噻唑基酰肼 (PTH) 能够抑制/分解 Tau 聚集体。然而,PTH 对 Tau 低聚物的解聚机制尚不清楚。在这项工作中,我们研究了 Tau 基序306 VQIVYK 311寡聚形式的破坏PTH 通过分子对接、分子动力学和自由能计算。我们预测疏水相互作用是 Tau 低聚物稳定的主要驱动力,其中 V306 和 I308 是主要贡献者。结合自由能的非极性成分对于稳定 Tau-PTH 复合物至关重要。PTH 主要破坏单体之间的范德华相互作用,导致低聚物不稳定。在采样的 20 ns 中没有观察到 PTH 和大黄素对全 Tau 丝的不稳定;然而,在所有情况下,结合自由能的非极性成分对于 Tau 丝-PTH 和 Tau 丝-大黄素的形成至关重要。这些结果为设计更有效的 Tau 聚集抑制剂提供了有用的线索。
更新日期:2020-03-30
中文翻译:

了解苯基噻唑基酰肼抑制剂对 Tau 聚集基序“306 VQIVYK311”的破坏机制。
阿尔茨海默病是一种进行性神经退行性疾病,其特征在于 Tau 和淀粉样前体蛋白的异常加工。Tau 的异常聚集基于通过两个基序形成分子间 β-折叠:275 VQIINK 280和306 VQIVYK 311。苯基噻唑基酰肼 (PTH) 能够抑制/分解 Tau 聚集体。然而,PTH 对 Tau 低聚物的解聚机制尚不清楚。在这项工作中,我们研究了 Tau 基序306 VQIVYK 311寡聚形式的破坏PTH 通过分子对接、分子动力学和自由能计算。我们预测疏水相互作用是 Tau 低聚物稳定的主要驱动力,其中 V306 和 I308 是主要贡献者。结合自由能的非极性成分对于稳定 Tau-PTH 复合物至关重要。PTH 主要破坏单体之间的范德华相互作用,导致低聚物不稳定。在采样的 20 ns 中没有观察到 PTH 和大黄素对全 Tau 丝的不稳定;然而,在所有情况下,结合自由能的非极性成分对于 Tau 丝-PTH 和 Tau 丝-大黄素的形成至关重要。这些结果为设计更有效的 Tau 聚集抑制剂提供了有用的线索。



























 京公网安备 11010802027423号
京公网安备 11010802027423号