当前位置:
X-MOL 学术
›
Phys. Chem. Chem. Phys.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Prediction of aqueous free energies of solvation using coupled QM and MM explicit solvent simulations.
Physical Chemistry Chemical Physics ( IF 3.3 ) Pub Date : 2020-04-02 , DOI: 10.1039/d0cp00582g Daniel Sadowsky 1 , J Samuel Arey 2
Physical Chemistry Chemical Physics ( IF 3.3 ) Pub Date : 2020-04-02 , DOI: 10.1039/d0cp00582g Daniel Sadowsky 1 , J Samuel Arey 2
Affiliation
|
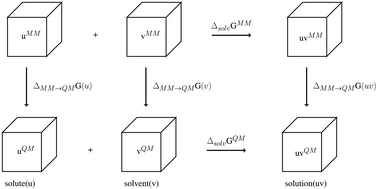
A method based on molecular dynamics simulations which employ two distinct levels of theory is proposed and tested for the prediction of Gibbs free energies of solvation for non-ionic solutes in water. The method consists of two additive contributions: (i) an evaluation of the free energy of solvation predicted by a computationally efficient molecular mechanics (MM) method; and (ii) an evaluation of the free energy difference between the potential energy surface of the MM method and that of a more computationally intensive first-principles quantum-mechanical (QM) method. The latter is computed by a thermodynamic integration method based on a series of shorter molecular dynamics simulations that employ weighted averages of the QM and MM force evaluations. The combined computational approach is tested against the experimental free energies of aqueous solvation for four solutes. For solute-solvent interactions that are found to be described qualitatively well by the MM method, the QM correction makes a modest improvement in the predicted free energy of aqueous solvation. However, for solutes that are found to not be adequately described by the MM method, the QM correction does not improve agreement with experiment. These preliminary results provide valuable insights into the novel concept of implementing thermodynamic integration between two model chemistries, suggesting that it is possible to use QM methods to improve upon the MM predictions of free energies of aqueous solvation.
中文翻译:

使用耦合的QM和MM显式溶剂模拟预测溶剂化的水溶液自由能。
提出了一种基于分子动力学模拟的方法,该方法采用了两种不同的理论水平,并通过该方法预测了水中非离子溶质的溶剂化吉布斯自由能。该方法由两个累加贡献组成:(i)通过计算有效的分子力学(MM)方法预测的溶剂化自由能的评估;(ii)评估MM方法的势能面与计算强度更高的第一原理量子力学(QM)方法的势能面之间的自由能差。后者是根据一系列较短的分子动力学模拟(通过采用QM和MM力评估的加权平均值)通过热力学积分方法计算得出的。针对四种溶质的水溶剂化实验自由能测试了组合计算方法。对于通过MM方法定性描述得很好的溶质-溶剂相互作用,QM校正在预测的水溶溶剂化自由能方面有适度的提高。但是,对于发现的MM方法不能充分描述的溶质,QM校正不能改善与实验的一致性。这些初步结果为实现两种模型化学之间的热力学整合提供了新颖的概念,从而提供了有价值的见解,这表明可以使用QM方法来改进MM对水性溶剂化自由能的预测。对于通过MM方法定性描述得很好的溶质-溶剂相互作用,QM校正在预测的水溶溶剂化自由能方面有适度的提高。但是,对于发现的MM方法不能充分描述的溶质,QM校正不能改善与实验的一致性。这些初步结果为实现两种模型化学之间的热力学整合提供了新颖的概念,从而提供了有价值的见解,这表明可以使用QM方法来改进MM对水性溶剂化自由能的预测。对于通过MM方法定性描述得很好的溶质-溶剂相互作用,QM校正在预测的水溶溶剂化自由能方面有适度的提高。但是,对于发现的MM方法不能充分描述的溶质,QM校正不能改善与实验的一致性。这些初步结果为实现两种模型化学之间的热力学整合提供了新颖的概念,从而提供了有价值的见解,这表明可以使用QM方法来改进MM对水溶液溶剂化自由能的预测。QM校正不能改善与实验的一致性。这些初步结果为实现两种模型化学之间的热力学整合提供了新颖的概念,从而提供了有价值的见解,这表明可以使用QM方法来改进MM对水性溶剂化自由能的预测。QM校正不能改善与实验的一致性。这些初步结果为实现两个模型化学之间的热力学整合提供了新颖的概念,从而提供了有价值的见解,表明有可能使用QM方法来改进MM对水溶剂化自由能的预测。
更新日期:2020-04-15
中文翻译:

使用耦合的QM和MM显式溶剂模拟预测溶剂化的水溶液自由能。
提出了一种基于分子动力学模拟的方法,该方法采用了两种不同的理论水平,并通过该方法预测了水中非离子溶质的溶剂化吉布斯自由能。该方法由两个累加贡献组成:(i)通过计算有效的分子力学(MM)方法预测的溶剂化自由能的评估;(ii)评估MM方法的势能面与计算强度更高的第一原理量子力学(QM)方法的势能面之间的自由能差。后者是根据一系列较短的分子动力学模拟(通过采用QM和MM力评估的加权平均值)通过热力学积分方法计算得出的。针对四种溶质的水溶剂化实验自由能测试了组合计算方法。对于通过MM方法定性描述得很好的溶质-溶剂相互作用,QM校正在预测的水溶溶剂化自由能方面有适度的提高。但是,对于发现的MM方法不能充分描述的溶质,QM校正不能改善与实验的一致性。这些初步结果为实现两种模型化学之间的热力学整合提供了新颖的概念,从而提供了有价值的见解,这表明可以使用QM方法来改进MM对水性溶剂化自由能的预测。对于通过MM方法定性描述得很好的溶质-溶剂相互作用,QM校正在预测的水溶溶剂化自由能方面有适度的提高。但是,对于发现的MM方法不能充分描述的溶质,QM校正不能改善与实验的一致性。这些初步结果为实现两种模型化学之间的热力学整合提供了新颖的概念,从而提供了有价值的见解,这表明可以使用QM方法来改进MM对水性溶剂化自由能的预测。对于通过MM方法定性描述得很好的溶质-溶剂相互作用,QM校正在预测的水溶溶剂化自由能方面有适度的提高。但是,对于发现的MM方法不能充分描述的溶质,QM校正不能改善与实验的一致性。这些初步结果为实现两种模型化学之间的热力学整合提供了新颖的概念,从而提供了有价值的见解,这表明可以使用QM方法来改进MM对水溶液溶剂化自由能的预测。QM校正不能改善与实验的一致性。这些初步结果为实现两种模型化学之间的热力学整合提供了新颖的概念,从而提供了有价值的见解,这表明可以使用QM方法来改进MM对水性溶剂化自由能的预测。QM校正不能改善与实验的一致性。这些初步结果为实现两个模型化学之间的热力学整合提供了新颖的概念,从而提供了有价值的见解,表明有可能使用QM方法来改进MM对水溶剂化自由能的预测。


























 京公网安备 11010802027423号
京公网安备 11010802027423号