当前位置:
X-MOL 学术
›
Phys. Chem. Chem. Phys.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Can glycine betaine denature proteins?
Physical Chemistry Chemical Physics ( IF 3.3 ) Pub Date : 2020-04-15 , DOI: 10.1039/d0cp00397b Arusha Acharyya 1 , Dayoung Shin 1 , Thomas Troxler 1 , Feng Gai 1
Physical Chemistry Chemical Physics ( IF 3.3 ) Pub Date : 2020-04-15 , DOI: 10.1039/d0cp00397b Arusha Acharyya 1 , Dayoung Shin 1 , Thomas Troxler 1 , Feng Gai 1
Affiliation
|
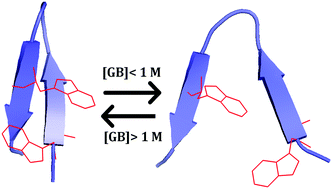
Glycine betaine (GB) is a naturally occurring osmolyte that has been widely recognized as a protein protectant. Since GB consists of a methylated ammonium moiety, it can engage in strong cation-π interactions with aromatic amino acid sidechains. We hypothesize that such specific binding interactions would allow GB to decrease the stability of proteins that are predominantly stabilized by a cluster of aromatic amino acids. To test this hypothesis, we investigate the effect of GB on the stability of two β-hairpins (or mini-proteins) that contain such a cluster. We find that for both systems the stability of the folded state first decreases and then increases with increasing GB concentration. Such non-monotonic dependence not only confirms that GB can act as a protein denaturant, but also underscores the complex interplay between GB's stabilizing and destabilizing forces toward a given protein. While stabilizing osmolytes all have the tendency to be excluded from the protein surface which is the action underlying their stabilizing effect, our results suggest that in order to quantitatively assess the effect of GB on the stability of any given protein, specific cation-π binding interactions need to be explicitly considered. Moreover, our results show, consistent with other studies, that cation methylation can strengthen the respective cation-π interactions. Taken together, these findings provide new insight into the mechanism by which amino acid-based osmolytes interact with proteins.
中文翻译:

甘氨酸甜菜碱能否使蛋白质变性?
甘氨酸甜菜碱(GB)是一种天然存在的渗透压剂,已被广泛认为是一种蛋白质保护剂。由于GB由甲基化的铵部分组成,因此它可以与芳香族氨基酸侧链发生强烈的阳离子-π相互作用。我们假设这样的特异性结合相互作用将使GB降低主要由芳香族氨基酸簇稳定的蛋白质的稳定性。为了验证这一假设,我们研究了GB对两个含有这种簇的β-发夹(或微型蛋白)稳定性的影响。我们发现,对于这两个系统,折叠态的稳定性首先随着GB浓度的增加而降低,然后增加。这种非单调的依赖性不仅证实了GB可以充当蛋白质变性剂,还强调了GB'之间的复杂相互作用。对给定蛋白质的稳定力和去稳定力。尽管稳定的渗透物都有被从蛋白表面排除的趋势,这是其稳定作用的基础,但我们的结果表明,为了定量评估GB对任何给定蛋白质的稳定性的作用,特定的阳离子-π结合相互作用需要明确考虑。而且,我们的结果表明,与其他研究一致,阳离子甲基化可以增强各自的阳离子-π相互作用。综上所述,这些发现为基于氨基酸的渗透物与蛋白质相互作用的机理提供了新的见解。我们的结果表明,为了定量评估GB对任何给定蛋白质的稳定性的影响,需要明确考虑特定的阳离子-π结合相互作用。而且,我们的结果表明,与其他研究一致,阳离子甲基化可以增强各自的阳离子-π相互作用。综上所述,这些发现为基于氨基酸的渗透物与蛋白质相互作用的机理提供了新的见解。我们的结果表明,为了定量评估GB对任何给定蛋白质的稳定性的影响,需要明确考虑特定的阳离子-π结合相互作用。而且,我们的结果表明,与其他研究一致,阳离子甲基化可以增强各自的阳离子-π相互作用。综上所述,这些发现为基于氨基酸的渗透物与蛋白质相互作用的机理提供了新的见解。
更新日期:2020-04-15
中文翻译:

甘氨酸甜菜碱能否使蛋白质变性?
甘氨酸甜菜碱(GB)是一种天然存在的渗透压剂,已被广泛认为是一种蛋白质保护剂。由于GB由甲基化的铵部分组成,因此它可以与芳香族氨基酸侧链发生强烈的阳离子-π相互作用。我们假设这样的特异性结合相互作用将使GB降低主要由芳香族氨基酸簇稳定的蛋白质的稳定性。为了验证这一假设,我们研究了GB对两个含有这种簇的β-发夹(或微型蛋白)稳定性的影响。我们发现,对于这两个系统,折叠态的稳定性首先随着GB浓度的增加而降低,然后增加。这种非单调的依赖性不仅证实了GB可以充当蛋白质变性剂,还强调了GB'之间的复杂相互作用。对给定蛋白质的稳定力和去稳定力。尽管稳定的渗透物都有被从蛋白表面排除的趋势,这是其稳定作用的基础,但我们的结果表明,为了定量评估GB对任何给定蛋白质的稳定性的作用,特定的阳离子-π结合相互作用需要明确考虑。而且,我们的结果表明,与其他研究一致,阳离子甲基化可以增强各自的阳离子-π相互作用。综上所述,这些发现为基于氨基酸的渗透物与蛋白质相互作用的机理提供了新的见解。我们的结果表明,为了定量评估GB对任何给定蛋白质的稳定性的影响,需要明确考虑特定的阳离子-π结合相互作用。而且,我们的结果表明,与其他研究一致,阳离子甲基化可以增强各自的阳离子-π相互作用。综上所述,这些发现为基于氨基酸的渗透物与蛋白质相互作用的机理提供了新的见解。我们的结果表明,为了定量评估GB对任何给定蛋白质的稳定性的影响,需要明确考虑特定的阳离子-π结合相互作用。而且,我们的结果表明,与其他研究一致,阳离子甲基化可以增强各自的阳离子-π相互作用。综上所述,这些发现为基于氨基酸的渗透物与蛋白质相互作用的机理提供了新的见解。



























 京公网安备 11010802027423号
京公网安备 11010802027423号