当前位置:
X-MOL 学术
›
Mol. Ther. Methods Clin. Dev.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Preclinical Toxicology of rQNestin34.5v.2: An Oncolytic Herpes Virus with Transcriptional Regulation of the ICP34.5 Neurovirulence Gene.
Molecular Therapy - Methods & Clinical Development ( IF 4.7 ) Pub Date : 2020-03-30 , DOI: 10.1016/j.omtm.2020.03.028 E Antonio Chiocca 1 , Hiroshi Nakashima 1 , Kazue Kasai 1 , Soledad A Fernandez 2 , Michael Oglesbee 3
Molecular Therapy - Methods & Clinical Development ( IF 4.7 ) Pub Date : 2020-03-30 , DOI: 10.1016/j.omtm.2020.03.028 E Antonio Chiocca 1 , Hiroshi Nakashima 1 , Kazue Kasai 1 , Soledad A Fernandez 2 , Michael Oglesbee 3
Affiliation
|
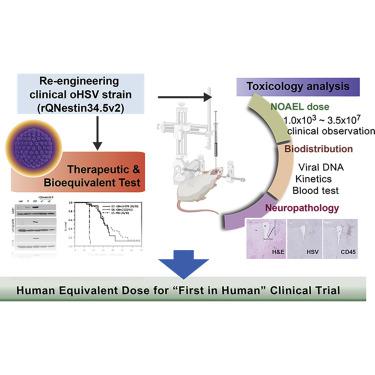
rQNestin34.5v.2 is an oncolytic herpes simplex virus 1 (oHSV) that retains expression of the neurovirulent ICP34.5 gene under glioma-selective transcriptional regulation. To prepare an investigational new drug (IND) application, we performed toxicology and efficacy studies of rQNestin34.5v.2 in mice in the presence or absence of the immunomodulating drug cyclophosphamide (CPA). ICP34.5 allows HSV1 to survive interferon and improves viral replication by dephosphorylation of the eIF-2α translation factor. rQNestin34.5v.2 dephosphorylated eIF-2α in human glioma cells, but not in human normal cells, resulting in significantly higher cytotoxicity and viral replication in the former compared to the latter. In vivo toxicity of rQNestin34.5v.2 was compared with that of wild-type F strain in immunocompetent BALB/c mice and athymic mice by multiple routes of administration in the presence or absence of CPA. A likely no observed adverse effect level (NOAEL) dose for intracranial rQNestin34.5v.2 was estimated, justifying a phase 1 clinical trial in recurrent glioma patients (ClinicalTrials.gov: NCT03152318), after successful submission of an IND.
中文翻译:

rQNestin34.5v.2的临床前毒理学:ICP34.5神经毒力基因转录调控的溶瘤性疱疹病毒。
rQNestin34.5v.2是一种溶瘤性单纯疱疹病毒1(oHSV),在神经胶质瘤选择性转录调控下保留了神经毒性ICP34.5基因的表达。为了准备研究性新药(IND)的应用,我们在存在或不存在免疫调节药物环磷酰胺(CPA)的情况下,在小鼠中进行了rQNestin34.5v.2的毒理学和功效研究。ICP34.5允许HSV1在干扰素中存活,并通过eIF-2α翻译因子的去磷酸化来改善病毒复制。rQNestin34.5v.2在人神经胶质瘤细胞中使eIF-2α去磷酸化,但在人正常细胞中没有,因此与后者相比,前者的细胞毒性和病毒复制明显更高。rQNestin34.5v的体内毒性。在存在或不存在CPA的情况下,通过多种给药途径,将2与野生型F株在具有免疫能力的BALB / c小鼠和无胸腺小鼠中进行比较。据估计,成功提交IND后,颅内rQNestin34.5v.2可能没有观察到的不良反应水平(NOAEL)剂量,证明对复发性神经胶质瘤患者进行了1期临床试验(ClinicalTrials.gov:NCT03152318)。
更新日期:2020-03-30
中文翻译:

rQNestin34.5v.2的临床前毒理学:ICP34.5神经毒力基因转录调控的溶瘤性疱疹病毒。
rQNestin34.5v.2是一种溶瘤性单纯疱疹病毒1(oHSV),在神经胶质瘤选择性转录调控下保留了神经毒性ICP34.5基因的表达。为了准备研究性新药(IND)的应用,我们在存在或不存在免疫调节药物环磷酰胺(CPA)的情况下,在小鼠中进行了rQNestin34.5v.2的毒理学和功效研究。ICP34.5允许HSV1在干扰素中存活,并通过eIF-2α翻译因子的去磷酸化来改善病毒复制。rQNestin34.5v.2在人神经胶质瘤细胞中使eIF-2α去磷酸化,但在人正常细胞中没有,因此与后者相比,前者的细胞毒性和病毒复制明显更高。rQNestin34.5v的体内毒性。在存在或不存在CPA的情况下,通过多种给药途径,将2与野生型F株在具有免疫能力的BALB / c小鼠和无胸腺小鼠中进行比较。据估计,成功提交IND后,颅内rQNestin34.5v.2可能没有观察到的不良反应水平(NOAEL)剂量,证明对复发性神经胶质瘤患者进行了1期临床试验(ClinicalTrials.gov:NCT03152318)。



























 京公网安备 11010802027423号
京公网安备 11010802027423号