当前位置:
X-MOL 学术
›
Food Chem. Toxicol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Liver toxicity of anthraquinones: A combined in vitro cytotoxicity and in silico reverse dosimetry evaluation.
Food and Chemical Toxicology ( IF 4.3 ) Pub Date : 2020-03-30 , DOI: 10.1016/j.fct.2020.111313 Yitong Liu 1 , Mapa S T Mapa 1 , Robert L Sprando 1
Food and Chemical Toxicology ( IF 4.3 ) Pub Date : 2020-03-30 , DOI: 10.1016/j.fct.2020.111313 Yitong Liu 1 , Mapa S T Mapa 1 , Robert L Sprando 1
Affiliation
|
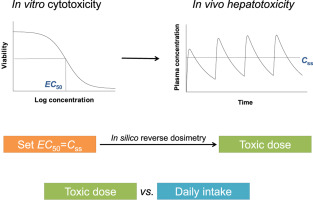
Anthraquinones are found in a variety of consumer products such as dietary supplements, traditional Chinese medicines, and drugs. Along with their widespread use, potential safety concerns have emerged, especially liver toxicity. Therefore, there is a need to conduct rapid and inexpensive safety assessment for anthraquinones due to a lack of animal and human toxicological data. Here, a combined in vitro cytotoxicity and in silico reverse dosimetry approach was adopted to consider the potential human liver toxicity of 16 anthraquinones and derivatives. First, cytotoxicity (EC50) in two human liver cell lines (HepG2/C3A and HuH-7) was measured under two conditions (single and repeated dosing, 72 h). Second, toxic doses (Dtox) required to yield plasma steady-state concentrations (Css) equal to in vitro EC50 values were predicted by reverse dosimetry simulation using a PBPK model. Finally, Dtox was compared to literature-derived estimated daily intake (EDI) of anthraquinones to assess safety. Among the 16 anthraquinones, rhein was identified as a potential hepatotoxicant due to a combination of cytotoxicity, plasma concentration, and daily intake level. These in vitro and in silico findings provide preliminary data and guidance for further animal and clinical studies to confirm liver toxicity of anthraquinones.
中文翻译:

蒽醌的肝毒性:体外细胞毒性和计算机反向剂量学评估相结合。
蒽醌存在于多种消费产品中,例如膳食补充剂,中药和药物。随着它们的广泛使用,出现了潜在的安全隐患,尤其是肝脏毒性。因此,由于缺乏动物和人类毒理学数据,需要对蒽醌进行快速而廉价的安全性评估。在这里,结合体外细胞毒性和计算机反向剂量测定法,考虑了16种蒽醌及其衍生物对人肝的潜在毒性。首先,在两种条件下(单次和重复给药,72小时)测量了两种人类肝细胞系(HepG2 / C3A和HuH-7)的细胞毒性(EC50)。第二,使用PBPK模型通过反向剂量法模拟可预测产生等于体外EC50值的血浆稳态浓度(Css)所需的毒性剂量(Dtox)。最后,将Dtox与蒽醌的文献估计每日摄入量(EDI)进行比较,以评估安全性。在16种蒽醌中,由于细胞毒性,血浆浓度和每日摄入量的结合,大黄酸被认为是潜在的肝毒性药物。这些体外和计算机上的发现为进一步的动物和临床研究提供初步数据和指导,以确认蒽醌的肝毒性。大黄酸由于细胞毒性,血浆浓度和每日摄入水平的组合而被鉴定为潜在的肝毒性药物。这些体外和计算机上的发现为进一步的动物和临床研究提供初步数据和指导,以确认蒽醌的肝毒性。大黄酸由于细胞毒性,血浆浓度和每日摄入水平的组合而被鉴定为潜在的肝毒性药物。这些体外和计算机模拟结果为进一步的动物和临床研究提供初步数据和指导,以确认蒽醌的肝毒性。
更新日期:2020-03-31
中文翻译:

蒽醌的肝毒性:体外细胞毒性和计算机反向剂量学评估相结合。
蒽醌存在于多种消费产品中,例如膳食补充剂,中药和药物。随着它们的广泛使用,出现了潜在的安全隐患,尤其是肝脏毒性。因此,由于缺乏动物和人类毒理学数据,需要对蒽醌进行快速而廉价的安全性评估。在这里,结合体外细胞毒性和计算机反向剂量测定法,考虑了16种蒽醌及其衍生物对人肝的潜在毒性。首先,在两种条件下(单次和重复给药,72小时)测量了两种人类肝细胞系(HepG2 / C3A和HuH-7)的细胞毒性(EC50)。第二,使用PBPK模型通过反向剂量法模拟可预测产生等于体外EC50值的血浆稳态浓度(Css)所需的毒性剂量(Dtox)。最后,将Dtox与蒽醌的文献估计每日摄入量(EDI)进行比较,以评估安全性。在16种蒽醌中,由于细胞毒性,血浆浓度和每日摄入量的结合,大黄酸被认为是潜在的肝毒性药物。这些体外和计算机上的发现为进一步的动物和临床研究提供初步数据和指导,以确认蒽醌的肝毒性。大黄酸由于细胞毒性,血浆浓度和每日摄入水平的组合而被鉴定为潜在的肝毒性药物。这些体外和计算机上的发现为进一步的动物和临床研究提供初步数据和指导,以确认蒽醌的肝毒性。大黄酸由于细胞毒性,血浆浓度和每日摄入水平的组合而被鉴定为潜在的肝毒性药物。这些体外和计算机模拟结果为进一步的动物和临床研究提供初步数据和指导,以确认蒽醌的肝毒性。



























 京公网安备 11010802027423号
京公网安备 11010802027423号