当前位置:
X-MOL 学术
›
Bioorgan. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Synthesis of N-substituted indole derivatives as potential antimicrobial and antileishmanial agents.
Bioorganic Chemistry ( IF 5.1 ) Pub Date : 2020-03-30 , DOI: 10.1016/j.bioorg.2020.103787 Shweta Tiwari 1 , Seema Kirar 2 , Uttam Chand Banerjee 2 , Kishore Babu Neerupudi 3 , Sushma Singh 3 , Aabid Abdullah Wani 4 , Prasad V Bharatam 4 , Inder Pal Singh 1
Bioorganic Chemistry ( IF 5.1 ) Pub Date : 2020-03-30 , DOI: 10.1016/j.bioorg.2020.103787 Shweta Tiwari 1 , Seema Kirar 2 , Uttam Chand Banerjee 2 , Kishore Babu Neerupudi 3 , Sushma Singh 3 , Aabid Abdullah Wani 4 , Prasad V Bharatam 4 , Inder Pal Singh 1
Affiliation
|
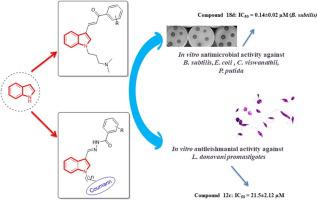
Leishmaniasis and microbial infections are two of the major contributors to global mortality and morbidity rates. Hence, development of novel, effective and safer antileishmanial and antimicrobial agents having reduced side effects are major priority for researchers. Two series of N-substituted indole derivatives i.e. N-substituted indole based chalcones (12a-g) and N-substituted indole based hydrazide-hydrazones (18a-g, 19a-f, 21 a-g) were synthesized. The synthesized compounds were characterized by 1H NMR, 13C NMR, Mass and FT-IR spectral data. Further these derivatives were evaluated for their antimicrobial potential against Escherichia coli, Bacillus subtilis, Pseudomonas putida and Candida viswanathii, and antileishmanial potential against promastigotes of Leishmania donovani. Compounds 18b, 18d and 19d exhibited significant activity with an IC50 of 0.19 ± 0.03 µM, 0.14 ± 0.02 µM and 0.16 ± 0.06 µM against B. subtilis which was comparable to chloramphenicol (IC50 of 0.25 ± 0.03 µM). Compounds 12b and 12c exhibited an IC50 of 24.2 ± 3.5 µM and 21.5 ± 2.1 µM in the antileishmanial assay. Binding interactions of indole based hydrazide-hydrazones were studied with nitric oxide synthase in silico in order to understand the structural features responsible for activity.
中文翻译:

N-取代的吲哚衍生物的合成作为潜在的抗菌和抗霉菌剂。
利什曼病和微生物感染是导致全球死亡率和发病率的两个主要因素。因此,开发具有减少的副作用的新颖,有效和安全的抗真菌药和抗微生物剂是研究人员的首要任务。合成了两个系列的N-取代的吲哚衍生物,即N-取代的基于吲哚的查耳酮(12a-g)和N-取代的基于吲哚的酰肼-azo(18a-g,19a-f,21ag)。合成的化合物通过1 H NMR,13 C NMR,质量和FT-IR光谱数据表征。进一步评估了这些衍生物的抗大肠杆菌,枯草芽孢杆菌,恶臭假单胞菌和粘链假丝酵母的抗微生物潜力,以及抗利什曼原虫的前鞭毛体的抗疟药潜力。化合物18b,18d和19d表现出显着的活性,对枯草芽孢杆菌的IC50为0.19±0.03 µM,0.14±0.02 µM和0.16±0.06 µM,与氯霉素相当(IC50为0.25±0.03 µM)。化合物12b和12c在抗盲肠测定中表现出24.2±3.5 µM和21.5±2.1 µM的IC50。为了研究负责活性的结构特征,研究了基于吲哚的酰肼-hydr与一氧化氮合酶在计算机上的结合相互作用。
更新日期:2020-04-20
中文翻译:

N-取代的吲哚衍生物的合成作为潜在的抗菌和抗霉菌剂。
利什曼病和微生物感染是导致全球死亡率和发病率的两个主要因素。因此,开发具有减少的副作用的新颖,有效和安全的抗真菌药和抗微生物剂是研究人员的首要任务。合成了两个系列的N-取代的吲哚衍生物,即N-取代的基于吲哚的查耳酮(12a-g)和N-取代的基于吲哚的酰肼-azo(18a-g,19a-f,21ag)。合成的化合物通过1 H NMR,13 C NMR,质量和FT-IR光谱数据表征。进一步评估了这些衍生物的抗大肠杆菌,枯草芽孢杆菌,恶臭假单胞菌和粘链假丝酵母的抗微生物潜力,以及抗利什曼原虫的前鞭毛体的抗疟药潜力。化合物18b,18d和19d表现出显着的活性,对枯草芽孢杆菌的IC50为0.19±0.03 µM,0.14±0.02 µM和0.16±0.06 µM,与氯霉素相当(IC50为0.25±0.03 µM)。化合物12b和12c在抗盲肠测定中表现出24.2±3.5 µM和21.5±2.1 µM的IC50。为了研究负责活性的结构特征,研究了基于吲哚的酰肼-hydr与一氧化氮合酶在计算机上的结合相互作用。



























 京公网安备 11010802027423号
京公网安备 11010802027423号