Waste Management ( IF 8.1 ) Pub Date : 2020-03-30 , DOI: 10.1016/j.wasman.2020.03.021 E. Torres-García , L.F. Ramírez-Verduzco , J. Aburto
|
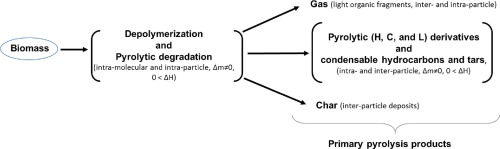
This study focuses on the thermo-kinetic analysis of solid peanut shell waste, through dependence of the activation energy with the conversion degree. Three model-free kinetics, Kissinger (K), Friedman (Fr) and Kissinger-Akahira-Sunose (KAS), were applied to thermogravimetric (TGA) data to calculate the effective activation energy Eα during a pyrolysis process. The results obtained by Kissinger’s method showed that the pyrolytic breakdown pathway of hemicellulose, cellulose, and lignin in a ligno-cellulosic biomass is independent of the heating rate and can be described through a simple first-order kinetic reaction (f(α) = 1 − α). The thermo-kinetic behavior obtained by isoconversional methods (Fr and KAS) of the hemicellulose degradation shows a progressive and monotonic increase in Eα with the conversion, between ~140 and ~195 kJ mol−1 with an average value of 172 kJ mol−1, which reveals the competitive character of the process (multi-step process). Conversely, in the cellulose degradation, the dependence of Eα on α, shows the typical behavior of a reaction controlled by a single rate-determining step, with constant average Eα values of ~209 kJ mol−1. Meanwhile, the lignin pyrolytic degradation shows an increase in Eα from ~220 up to ~300 kJ mol−1 with the conversion, indicating that this stage is kinetically controlled by an energy barrier that comprises multiple and simultaneous processes. Finally, the kinetic analysis confirmed the absence of autocatalytic reactions (thermally auto-catalyzed process) during the pyrolysis, although the global process is highly exothermic.
中文翻译:

花生壳的热解降解:活化能与转化率的关系
这项研究的重点是通过活化能与转化度的关系来分析固体花生壳废料的热动力学。三个无模型动力学,基辛格(K),弗里德曼(FR)和基辛格-赤平-Sunose(KAS),分别适用于热重量(TGA)数据来计算有效活化能Ë α期间热解过程。基辛格方法获得的结果表明,木质纤维素生物质中半纤维素,纤维素和木质素的热分解途径与加热速率无关,可以通过简单的一级动力学反应(f(α)= 1) 来描述。−α)。通过的半纤维素降解示出了渐进的和单调增加在等转化率的方法(FR和KAS)中获得的热动力学行为Ë α与转换之间〜140和195〜千焦耳摩尔-1与172千焦耳摩尔的平均值-图1揭示了流程(多步骤流程)的竞争特征。相反,在纤维素降解,的依赖性ë α上α,示出了由一个单一的速率决定步骤所控制的反应,具有恒定的平均的典型行为ë α〜209千焦摩尔的值-1。同时,木质素的热分解显示出E的增加转化后, α从〜220高达〜300 kJ mol -1,表明该阶段由包括多个同时过程的能垒进行动力学控制。最后,动力学分析证实了热解过程中不存在自催化反应(热自催化过程),尽管总体过程是放热的。



























 京公网安备 11010802027423号
京公网安备 11010802027423号