当前位置:
X-MOL 学术
›
J. Hepatol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
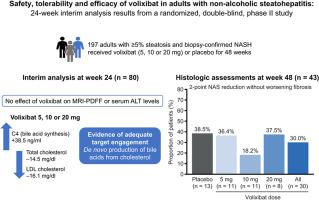
Volixibat in adults with non-alcoholic steatohepatitis: 24-week interim analysis from a randomized, phase II study
Journal of Hepatology ( IF 25.7 ) Pub Date : 2020-08-01 , DOI: 10.1016/j.jhep.2020.03.024 Philip N Newsome 1 , Melissa Palmer 2 , Bradley Freilich 3 , Muhammad Y Sheikh 4 , Aasim Sheikh 5 , Harry Sarles 6 , Robert Herring 7 , Parvez Mantry 8 , Zeid Kayali 9 , Tarek Hassanein 10 , Hak-Myung Lee 2 , Guruprasad P Aithal 11 ,
Journal of Hepatology ( IF 25.7 ) Pub Date : 2020-08-01 , DOI: 10.1016/j.jhep.2020.03.024 Philip N Newsome 1 , Melissa Palmer 2 , Bradley Freilich 3 , Muhammad Y Sheikh 4 , Aasim Sheikh 5 , Harry Sarles 6 , Robert Herring 7 , Parvez Mantry 8 , Zeid Kayali 9 , Tarek Hassanein 10 , Hak-Myung Lee 2 , Guruprasad P Aithal 11 ,
Affiliation
|
BCKGROUND & AIMS
Volixibat is an inhibitor of the apical sodium-dependent bile acid transporter (ASBT), hypothesized to treat non-alcoholic steatohepatitis (NASH) by blocking bile acid reuptake and stimulating hepatic bile acid production. METHODS
Adults with ≥5% steatosis and NASH without cirrhosis (N = 197) were randomized to receive double-blind volixibat 5, 10 or 20 mg or placebo once daily for 48 weeks. A predefined interim analysis (n = 80) at week 24 had endpoints of ≥5% reduction in magnetic resonance imaging-proton density fat fraction and ≥20% reduction in serum alanine aminotransferase levels. The primary endpoint was ≥2-point reduction in non-alcoholic fatty liver disease activity score without worsening fibrosis at week 48. RESULTS
Volixibat did not meet either interim endpoint; the study was terminated owing to lack of efficacy. In participants receiving any volixibat dose, mean serum 7-alpha-hydroxy-4-cholesten-3-one (C4; a biomarker of bile acid synthesis) increased from baseline to week 24 (+38.5 ng/mL [standard deviation (SD) 53.18]), with concomitant decreases in serum total cholesterol (-14.5 mg/dL [SD 28.32]) and low-density lipoprotein cholesterol (-16.1 mg/dL [SD 25.31]). These changes were generally dose-dependent. In the liver histology analysis, a greater proportion of participants receiving placebo (38.5%, n = 5/13) than volixibat (30.0%, n = 9/30) met the primary endpoint. Treatment-emergent adverse events (TEAEs) were mainly mild or moderate. No serious TEAEs were related to volixibat. Diarrhoea was the most common TEAE overall and the most common TEAE leading to discontinuation. CONCLUSIONS
Increased serum C4 and decreased serum cholesterol levels provide evidence of target engagement. However, there was no therapeutic benefit of ASBT inhibition with volixibat on the liver in adults with NASH (ClinicalTrials.gov identifier: NCT02787304).
中文翻译:

非酒精性脂肪性肝炎成人中的 Volixibat:来自随机 II 期研究的 24 周中期分析
BCKGROUND & AIMS Volixibat 是一种顶端钠依赖性胆汁酸转运蛋白 (ASBT) 的抑制剂,假设通过阻断胆汁酸再摄取和刺激肝胆汁酸生成来治疗非酒精性脂肪性肝炎 (NASH)。方法 脂肪变性≥5% 且无肝硬化 NASH 的成人(N = 197)随机接受双盲 volixibat 5、10 或 20 mg 或安慰剂,每天一次,持续 48 周。第 24 周预定义的中期分析 (n = 80) 的终点是磁共振成像质子密度脂肪分数降低 ≥ 5%,血清丙氨酸转氨酶水平降低 ≥ 20%。主要终点是第 48 周非酒精性脂肪性肝病活动评分降低 ≥ 2 分,但未出现纤维化恶化。结果 Volixibat 未达到任一中期终点;该研究因缺乏疗效而终止。在接受任何 volixibat 剂量的参与者中,平均血清 7-alpha-hydroxy-4-cholesten-3-one(C4;胆汁酸合成的生物标志物)从基线增加到第 24 周(+38.5 ng/mL [标准偏差 (SD) 53.18]),同时血清总胆固醇 (-14.5 mg/dL [SD 28.32]) 和低密度脂蛋白胆固醇 (-16.1 mg/dL [SD 25.31]) 降低。这些变化通常是剂量依赖性的。在肝脏组织学分析中,与 volixibat (30.0%,n = 9/30) 相比,接受安慰剂的参与者 (38.5%,n = 5/13) 达到主要终点的比例更高。治疗中出现的不良事件 (TEAE) 主要是轻度或中度的。没有严重的 TEAE 与 volixibat 相关。腹泻是总体上最常见的 TEAE,也是最常见的导致停药的 TEAE。结论 增加的血清 C4 和降低的血清胆固醇水平提供了目标参与的证据。然而,在患有 NASH 的成人中,使用 volixibat 抑制 ASBT 对肝脏没有治疗益处(ClinicalTrials.gov 标识符:NCT02787304)。
更新日期:2020-08-01
中文翻译:

非酒精性脂肪性肝炎成人中的 Volixibat:来自随机 II 期研究的 24 周中期分析
BCKGROUND & AIMS Volixibat 是一种顶端钠依赖性胆汁酸转运蛋白 (ASBT) 的抑制剂,假设通过阻断胆汁酸再摄取和刺激肝胆汁酸生成来治疗非酒精性脂肪性肝炎 (NASH)。方法 脂肪变性≥5% 且无肝硬化 NASH 的成人(N = 197)随机接受双盲 volixibat 5、10 或 20 mg 或安慰剂,每天一次,持续 48 周。第 24 周预定义的中期分析 (n = 80) 的终点是磁共振成像质子密度脂肪分数降低 ≥ 5%,血清丙氨酸转氨酶水平降低 ≥ 20%。主要终点是第 48 周非酒精性脂肪性肝病活动评分降低 ≥ 2 分,但未出现纤维化恶化。结果 Volixibat 未达到任一中期终点;该研究因缺乏疗效而终止。在接受任何 volixibat 剂量的参与者中,平均血清 7-alpha-hydroxy-4-cholesten-3-one(C4;胆汁酸合成的生物标志物)从基线增加到第 24 周(+38.5 ng/mL [标准偏差 (SD) 53.18]),同时血清总胆固醇 (-14.5 mg/dL [SD 28.32]) 和低密度脂蛋白胆固醇 (-16.1 mg/dL [SD 25.31]) 降低。这些变化通常是剂量依赖性的。在肝脏组织学分析中,与 volixibat (30.0%,n = 9/30) 相比,接受安慰剂的参与者 (38.5%,n = 5/13) 达到主要终点的比例更高。治疗中出现的不良事件 (TEAE) 主要是轻度或中度的。没有严重的 TEAE 与 volixibat 相关。腹泻是总体上最常见的 TEAE,也是最常见的导致停药的 TEAE。结论 增加的血清 C4 和降低的血清胆固醇水平提供了目标参与的证据。然而,在患有 NASH 的成人中,使用 volixibat 抑制 ASBT 对肝脏没有治疗益处(ClinicalTrials.gov 标识符:NCT02787304)。


























 京公网安备 11010802027423号
京公网安备 11010802027423号