当前位置:
X-MOL 学术
›
Eur. J. Med. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Hypoxia-activated nanomedicines for effective cancer therapy.
European Journal of Medicinal Chemistry ( IF 6.7 ) Pub Date : 2020-03-30 , DOI: 10.1016/j.ejmech.2020.112274 Mengjiao Zhou 1 , Yuqi Xie 1 , Shujun Xu 1 , Jingqi Xin 2 , Jin Wang 3 , Tao Han 4 , Richard Ting 5 , Jie Zhang 3 , Feifei An 2
European Journal of Medicinal Chemistry ( IF 6.7 ) Pub Date : 2020-03-30 , DOI: 10.1016/j.ejmech.2020.112274 Mengjiao Zhou 1 , Yuqi Xie 1 , Shujun Xu 1 , Jingqi Xin 2 , Jin Wang 3 , Tao Han 4 , Richard Ting 5 , Jie Zhang 3 , Feifei An 2
Affiliation
|
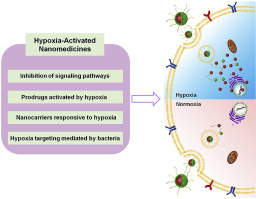
Hypoxia, a common characteristic in solid tumors, is found in phenotypically aggressive cancers that display resistance to typical cancer interventions. Due to its important role in tumor progression, tumor hypoxia has been considered as a primary target for cancer diagnosis and treatment. An advantage of hypoxia-activated nanomedicines is that they are inactive in normoxic cells. In hypoxic tumor tissues and cells, these nanomedicines undergo reduction by activated enzymes (usually through 1 or 2 electron oxidoreductases) to produce cytotoxic substances. In this review, we will focus on approaches to design nanomedicines that take advantage of tumor hypoxia. These approaches include: i) inhibitors of hypoxia-associated signaling pathways; ii) prodrugs activated by hypoxia; iii) nanocarriers responsive to hypoxia, and iv) bacteria mediated hypoxia targeting therapy. These strategies have guided and will continue to guide nanoparticle design in the near future. These strategies have the potential to overcome tumor heterogeneity to improve the efficiency of radiotherapy, chemotherapy and diagnosis.
中文翻译:

低氧激活的纳米药物可有效治疗癌症。
缺氧是实体瘤的常见特征,在表现出对典型癌症干预措施耐药性的表型侵袭性癌症中被发现。由于其在肿瘤进展中的重要作用,低氧被认为是癌症诊断和治疗的主要目标。缺氧激活的纳米药物的一个优势是它们在常氧细胞中无活性。在缺氧的肿瘤组织和细胞中,这些纳米药物会被活化酶(通常通过1或2个电子氧化还原酶)还原,从而产生细胞毒性物质。在这篇综述中,我们将重点研究利用肿瘤缺氧的纳米药物设计方法。这些方法包括:i)缺氧相关信号通路的抑制剂;ii)由缺氧激活的前药;iii)对缺氧有反应的纳米载体,iv)细菌介导的低氧靶向治疗。这些策略已经指导并将在不久的将来继续指导纳米颗粒设计。这些策略具有克服肿瘤异质性以提高放射疗法,化学疗法和诊断效率的潜力。
更新日期:2020-03-30
中文翻译:

低氧激活的纳米药物可有效治疗癌症。
缺氧是实体瘤的常见特征,在表现出对典型癌症干预措施耐药性的表型侵袭性癌症中被发现。由于其在肿瘤进展中的重要作用,低氧被认为是癌症诊断和治疗的主要目标。缺氧激活的纳米药物的一个优势是它们在常氧细胞中无活性。在缺氧的肿瘤组织和细胞中,这些纳米药物会被活化酶(通常通过1或2个电子氧化还原酶)还原,从而产生细胞毒性物质。在这篇综述中,我们将重点研究利用肿瘤缺氧的纳米药物设计方法。这些方法包括:i)缺氧相关信号通路的抑制剂;ii)由缺氧激活的前药;iii)对缺氧有反应的纳米载体,iv)细菌介导的低氧靶向治疗。这些策略已经指导并将在不久的将来继续指导纳米颗粒设计。这些策略具有克服肿瘤异质性以提高放射疗法,化学疗法和诊断效率的潜力。



























 京公网安备 11010802027423号
京公网安备 11010802027423号