当前位置:
X-MOL 学术
›
Chem. Res. Toxicol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Triiodothyronine Attenuates Silica-Induced Oxidative Stress, Inflammation, and Apoptosis via Thyroid Hormone Receptor α in Differentiated THP-1 Macrophages.
Chemical Research in Toxicology ( IF 4.1 ) Pub Date : 2020-04-03 , DOI: 10.1021/acs.chemrestox.0c00018 Shiming Gan 1, 2 , Meng Yang 1, 2 , Lieyang Fan 1, 2 , Li Xie 1, 2 , Yiju Xu 1, 2 , Bin Wang 1, 2 , Tao Xu 1, 2 , Linling Yu 1, 2 , Jixuan Ma 1, 2 , Weihong Chen 1, 2
Chemical Research in Toxicology ( IF 4.1 ) Pub Date : 2020-04-03 , DOI: 10.1021/acs.chemrestox.0c00018 Shiming Gan 1, 2 , Meng Yang 1, 2 , Lieyang Fan 1, 2 , Li Xie 1, 2 , Yiju Xu 1, 2 , Bin Wang 1, 2 , Tao Xu 1, 2 , Linling Yu 1, 2 , Jixuan Ma 1, 2 , Weihong Chen 1, 2
Affiliation
|
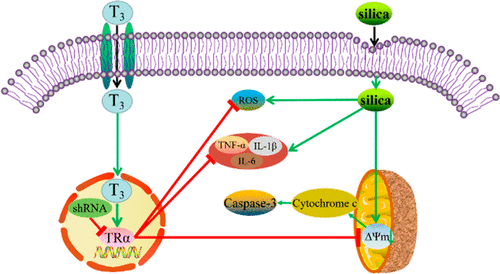
Alveolar macrophage (AM) injury and inflammatory response are key processes in pathological damage caused by silica. However, the role of triiodothyronine (T3) in silica-induced AM oxidative stress, inflammation, and mitochondrial apoptosis remained unknown. To investigate the possible effects and underlying mechanism of T3 in silica-induced macrophage damage, differentiated human acute monocytic leukemia cells (THP-1) were exposed to different silica concentrations (0, 50, 100, 200, and 400 μg/mL) for 24 h. Additionally, silica-activated THP-1 macrophages were treated with gradient-dose T3 (0, 5, 10, 20, and 40 nM) for 24 h. To illuminate the potential mechanism, we used short hairpin RNA to knock down the thyroid hormone receptor α (TRα) in the differentiated THP-1 macrophages. The results showed that T3 decreased lactate dehydrogenase and reactive oxygen species levels, while increasing cell viability and superoxide dismutase in silica-induced THP-1 macrophages. In addition, silica increased the expression of interleukin 1 beta (IL-1β), interleukin 6 (IL-6), and tumor necrosis factor-α (TNF-α), and T3 treatment reduced those pro-inflammatory cytokines secretion. Compared with silica-alone treated groups, cells treated with silica and T3 restored the mitochondrial membrane potential loss and had reduced levels of cytochrome c and cleaved caspase-3 expressions. Lastly, we observed that TRα-knockdown inhibited the protective effects of T3 silica-induced THP-1 macrophages. Together, these findings revealed that T3 could serve as a potential therapeutic target for protection against silica-induced oxidative stress, inflammatory response, and mitochondrial apoptosis, which are mediated by the activation of the T3/TRα signal pathway.
中文翻译:

Triiodothyronine通过甲状腺激素受体α在分化的THP-1巨噬细胞中减轻二氧化硅诱导的氧化应激,炎症和细胞凋亡。
肺泡巨噬细胞(AM)损伤和炎症反应是二氧化硅引起的病理损伤的关键过程。然而,三碘甲状腺氨酸(T3)在二氧化硅诱导的AM氧化应激,炎症和线粒体细胞凋亡中的作用仍然未知。为了研究T3在二氧化硅诱导的巨噬细胞损伤中的可能作用及其潜在机制,将分化的人类急性单核细胞白血病细胞(THP-1)暴露于不同的二氧化硅浓度(0、50、100、200和400μg/ mL)下, 24小时 另外,用梯度剂量的T3(0、5、10、20和40 nM)处理二氧化硅活化的THP-1巨噬细胞24小时。为了阐明潜在的机制,我们使用了短发夹RNA敲低分化的THP-1巨噬细胞中的甲状腺激素受体α(TRα)。结果表明,T3降低了乳酸诱导的THP-1巨噬细胞中的乳酸脱氢酶和活性氧水平,同时增加了细胞活力和超氧化物歧化酶。此外,二氧化硅可增加白介素1β(IL-1β),白介素6(IL-6)和肿瘤坏死因子-α(TNF-α)的表达,T3处理可减少促炎性细胞因子的分泌。与仅使用硅石处理的组相比,用硅石和T3处理的细胞恢复了线粒体膜电位损失,并降低了细胞色素c的水平和caspase-3的表达。最后,我们观察到TRα-knockdown抑制了T3二氧化硅诱导的THP-1巨噬细胞的保护作用。总之,这些发现表明T3可以作为潜在的治疗靶标来保护其免受氧化硅诱导的氧化应激的影响,
更新日期:2020-03-30
中文翻译:

Triiodothyronine通过甲状腺激素受体α在分化的THP-1巨噬细胞中减轻二氧化硅诱导的氧化应激,炎症和细胞凋亡。
肺泡巨噬细胞(AM)损伤和炎症反应是二氧化硅引起的病理损伤的关键过程。然而,三碘甲状腺氨酸(T3)在二氧化硅诱导的AM氧化应激,炎症和线粒体细胞凋亡中的作用仍然未知。为了研究T3在二氧化硅诱导的巨噬细胞损伤中的可能作用及其潜在机制,将分化的人类急性单核细胞白血病细胞(THP-1)暴露于不同的二氧化硅浓度(0、50、100、200和400μg/ mL)下, 24小时 另外,用梯度剂量的T3(0、5、10、20和40 nM)处理二氧化硅活化的THP-1巨噬细胞24小时。为了阐明潜在的机制,我们使用了短发夹RNA敲低分化的THP-1巨噬细胞中的甲状腺激素受体α(TRα)。结果表明,T3降低了乳酸诱导的THP-1巨噬细胞中的乳酸脱氢酶和活性氧水平,同时增加了细胞活力和超氧化物歧化酶。此外,二氧化硅可增加白介素1β(IL-1β),白介素6(IL-6)和肿瘤坏死因子-α(TNF-α)的表达,T3处理可减少促炎性细胞因子的分泌。与仅使用硅石处理的组相比,用硅石和T3处理的细胞恢复了线粒体膜电位损失,并降低了细胞色素c的水平和caspase-3的表达。最后,我们观察到TRα-knockdown抑制了T3二氧化硅诱导的THP-1巨噬细胞的保护作用。总之,这些发现表明T3可以作为潜在的治疗靶标来保护其免受氧化硅诱导的氧化应激的影响,



























 京公网安备 11010802027423号
京公网安备 11010802027423号