当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Dioxazolones: Stable Substrates for the Catalytic Transfer of Acyl Nitrenes
ACS Catalysis ( IF 12.9 ) Pub Date : 2020-03-30 , DOI: 10.1021/acscatal.0c00961 Kaj M. van Vliet 1 , Bas de Bruin 1
ACS Catalysis ( IF 12.9 ) Pub Date : 2020-03-30 , DOI: 10.1021/acscatal.0c00961 Kaj M. van Vliet 1 , Bas de Bruin 1
Affiliation
|
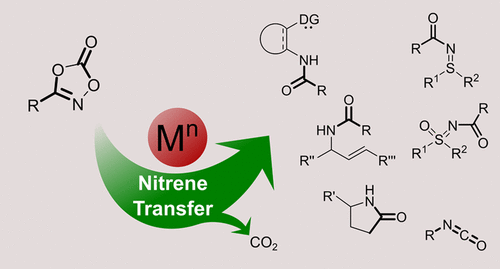
Dioxazolones are a convenient class of acyl nitrene transfer reagents. Their application in homogeneous transition-metal catalysis has led to many new amidation reactions. Dioxazolones are typically activated by transition metals at relatively low reaction temperatures. The metal nitrenoids formed by decarboxylative activation of dioxazolones are generally electron deficient and commonly react in a concerted fashion. “Intermolecular” nitrene insertion reactions involving preactivated C–H bonds (“inner-sphere” mechanism) easily compete with the Curtius-type rearrangement, but for intramolecular “direct” nitrene transfer/insertion reactions involving nonpreactivated substrates (i.e., without preceding formation of metal–carbon or metal–hydride bonds) extensive ligand optimization is important to prevent such unwanted side reactions. The ease of dioxazolone synthesis, formation of CO2 gas as the sole byproduct from reactions with dioxazolones, and the importance of nitrene transfer reactions in general has led to the development of several interesting reactions producing N-aryl amides, oxazoles, and lactams. Since the activation of dioxazolones proceeds under mild reaction conditions, stereo- and enantioselective reactions are also possible, which is useful for the synthesis of bioactive nitrogen-containing compounds. This review provides an overview of these reactions reported in recent years.
中文翻译:

二恶唑酮:稳定的底物的酰基硝基催化转移。
二恶唑酮是一类方便的酰基腈转移试剂。它们在均相过渡金属催化中的应用导致了许多新的酰胺化反应。二恶唑酮通常在较低的反应温度下被过渡金属活化。通过二恶唑酮的脱羧活化作用形成的金属亚硝酸酯通常是电子缺陷的,并且通常以协同方式反应。涉及预先活化的C–H键的“分子间”氮烯插入反应(“内部球”机制)很容易与Curtius型重排竞争,但对于涉及未预先活化的底物的分子内“直接”氮烯转移/插入反应(即,没有事先形成金属-碳或金属-氢键)广泛的配体优化对于防止此类不良副反应很重要。作为与二恶唑酮的反应的唯一副产物的2气体,以及一般来说,腈转移反应的重要性已导致开发了一些有趣的反应,产生N-芳基酰胺,恶唑和内酰胺。由于二恶唑酮的活化在温和的反应条件下进行,因此立体和对映选择性反应也是可能的,这对于合成生物活性含氮化合物是有用的。这篇综述概述了近年来报道的这些反应。
更新日期:2020-04-23
中文翻译:

二恶唑酮:稳定的底物的酰基硝基催化转移。
二恶唑酮是一类方便的酰基腈转移试剂。它们在均相过渡金属催化中的应用导致了许多新的酰胺化反应。二恶唑酮通常在较低的反应温度下被过渡金属活化。通过二恶唑酮的脱羧活化作用形成的金属亚硝酸酯通常是电子缺陷的,并且通常以协同方式反应。涉及预先活化的C–H键的“分子间”氮烯插入反应(“内部球”机制)很容易与Curtius型重排竞争,但对于涉及未预先活化的底物的分子内“直接”氮烯转移/插入反应(即,没有事先形成金属-碳或金属-氢键)广泛的配体优化对于防止此类不良副反应很重要。作为与二恶唑酮的反应的唯一副产物的2气体,以及一般来说,腈转移反应的重要性已导致开发了一些有趣的反应,产生N-芳基酰胺,恶唑和内酰胺。由于二恶唑酮的活化在温和的反应条件下进行,因此立体和对映选择性反应也是可能的,这对于合成生物活性含氮化合物是有用的。这篇综述概述了近年来报道的这些反应。



























 京公网安备 11010802027423号
京公网安备 11010802027423号