当前位置:
X-MOL 学术
›
Mol. Pharmaceutics
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Cell Type-Dependent Specificity and Anti-Inflammatory Effects of Charge-Reversible MSNs-COS-CMC for Targeted Drug Delivery in Cervical Carcinoma.
Molecular Pharmaceutics ( IF 4.9 ) Pub Date : 2020-03-30 , DOI: 10.1021/acs.molpharmaceut.0c00004 Lan Cui 1 , Xiayi Feng 2 , Wentao Liu 1 , Hao Liu 1 , Qian Qin 1, 3 , Shuangxia Wu 1 , Suqin He 1, 4 , Xinchang Pang 1 , Dong Men 2 , Chengshen Zhu 1
Molecular Pharmaceutics ( IF 4.9 ) Pub Date : 2020-03-30 , DOI: 10.1021/acs.molpharmaceut.0c00004 Lan Cui 1 , Xiayi Feng 2 , Wentao Liu 1 , Hao Liu 1 , Qian Qin 1, 3 , Shuangxia Wu 1 , Suqin He 1, 4 , Xinchang Pang 1 , Dong Men 2 , Chengshen Zhu 1
Affiliation
|
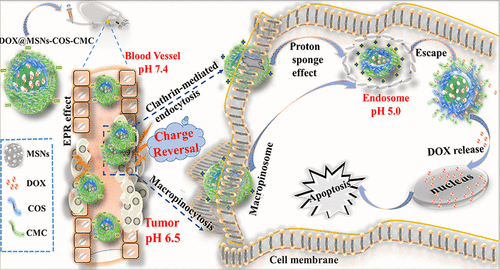
The surface charge of nanocarriers inevitably affects drug delivery efficiency; however, the cancer cell specificity, anti-inflammatory effects, and charge-reversal points remain to be further addressed in biomedical applications. The aim of this study was to comprehensively assess the cancer cell specificity of DOX-loaded mesoporous silica-chitosan oligosaccharide-carboxymethyl chitosan nanoparticles (DOX@MSNs-COS-CMC) in MCF-7 and HeLa cells, inhibit the production of inflammatory cytokines, and improve the drug accumulation in the tumor site. Intracellular results reveal that the retention time prolonged to 48 h in both HeLa and MCF-7 cells at pH 7.4. However, DOX@MSNs-COS-CMC exhibited a cell type-dependent cytotoxicity and enhanced intracellular uptake in HeLa cells at pH 6.5, due to the clathrin-mediated endocytosis and macropinocytosis in HeLa cells in comparison with the vesicular transport in MCF-7 cells. Moreover, Pearson’s correlation coefficient value significantly decreased to 0.25 after 8 h, prompting endosomal escape and drug delivery into the HeLa nucleus. After the treatment of MSNs-COS-CMC at 200 μg/mL, the inflammatory cytokines IL-6 and TNF-α level decreased by 70% and 80%, respectively. Tumor inhibition of DOX@MSNs-COS-CMC was 0.4 times higher than free DOX, alleviating cardiotoxicity and inflammation in the HeLa xenograft tumor model. Charge-reversible DOX@MSNs-COS-CMC could be a possible candidate for clinical therapy of cervical carcinoma.
中文翻译:

电荷可逆性MSNs-COS-CMC在宫颈癌中靶向药物递送的细胞类型依赖性特异性和抗炎作用。
纳米载体的表面电荷不可避免地影响药物传递效率。然而,癌细胞的特异性,抗炎作用和电荷逆转点在生物医学应用中仍有待进一步解决。这项研究的目的是全面评估MCF-7和HeLa细胞中负载DOX的介孔二氧化硅-壳聚糖寡糖-羧甲基壳聚糖纳米颗粒(DOX @ MSNs-COS-CMC)的癌细胞特异性,抑制炎症细胞因子的产生,并改善药物在肿瘤部位的蓄积。细胞内结果显示,在pH 7.4的HeLa和MCF-7细胞中,保留时间均延长至48小时。但是,DOX @ MSNs-COS-CMC在pH 6.5的HeLa细胞中表现出细胞类型依赖性的细胞毒性并增强了细胞内摄取,与在MCF-7细胞中的囊泡转运相比,是由于网格蛋白介导的HeLa细胞内吞作用和巨胞饮作用。此外,Pearson相关系数值在8 h后显着降低至0.25,促使内体逃逸并将药物输送到HeLa核中。用200μg/ mL的MSNs-COS-CMC处理后,炎性细胞因子IL-6和TNF-α水平分别降低了70%和80%。DOX @ MSNs-COS-CMC的肿瘤抑制作用比游离DOX高0.4倍,从而减轻了HeLa异种移植肿瘤模型的心脏毒性和炎症。可逆电荷的DOX @ MSNs-COS-CMC可能是宫颈癌临床治疗的候选药物。促使内体逃逸并将药物输送到HeLa核中。用200μg/ mL的MSNs-COS-CMC处理后,炎性细胞因子IL-6和TNF-α水平分别降低了70%和80%。DOX @ MSNs-COS-CMC的肿瘤抑制作用比游离DOX高0.4倍,从而减轻了HeLa异种移植肿瘤模型的心脏毒性和炎症。可逆电荷的DOX @ MSNs-COS-CMC可能是宫颈癌临床治疗的候选药物。促使内体逃逸并将药物输送到HeLa核中。用200μg/ mL的MSNs-COS-CMC处理后,炎性细胞因子IL-6和TNF-α水平分别降低了70%和80%。DOX @ MSNs-COS-CMC的肿瘤抑制作用比游离DOX高0.4倍,从而减轻了HeLa异种移植肿瘤模型的心脏毒性和炎症。可逆电荷的DOX @ MSNs-COS-CMC可能是宫颈癌临床治疗的候选药物。
更新日期:2020-03-30
中文翻译:

电荷可逆性MSNs-COS-CMC在宫颈癌中靶向药物递送的细胞类型依赖性特异性和抗炎作用。
纳米载体的表面电荷不可避免地影响药物传递效率。然而,癌细胞的特异性,抗炎作用和电荷逆转点在生物医学应用中仍有待进一步解决。这项研究的目的是全面评估MCF-7和HeLa细胞中负载DOX的介孔二氧化硅-壳聚糖寡糖-羧甲基壳聚糖纳米颗粒(DOX @ MSNs-COS-CMC)的癌细胞特异性,抑制炎症细胞因子的产生,并改善药物在肿瘤部位的蓄积。细胞内结果显示,在pH 7.4的HeLa和MCF-7细胞中,保留时间均延长至48小时。但是,DOX @ MSNs-COS-CMC在pH 6.5的HeLa细胞中表现出细胞类型依赖性的细胞毒性并增强了细胞内摄取,与在MCF-7细胞中的囊泡转运相比,是由于网格蛋白介导的HeLa细胞内吞作用和巨胞饮作用。此外,Pearson相关系数值在8 h后显着降低至0.25,促使内体逃逸并将药物输送到HeLa核中。用200μg/ mL的MSNs-COS-CMC处理后,炎性细胞因子IL-6和TNF-α水平分别降低了70%和80%。DOX @ MSNs-COS-CMC的肿瘤抑制作用比游离DOX高0.4倍,从而减轻了HeLa异种移植肿瘤模型的心脏毒性和炎症。可逆电荷的DOX @ MSNs-COS-CMC可能是宫颈癌临床治疗的候选药物。促使内体逃逸并将药物输送到HeLa核中。用200μg/ mL的MSNs-COS-CMC处理后,炎性细胞因子IL-6和TNF-α水平分别降低了70%和80%。DOX @ MSNs-COS-CMC的肿瘤抑制作用比游离DOX高0.4倍,从而减轻了HeLa异种移植肿瘤模型的心脏毒性和炎症。可逆电荷的DOX @ MSNs-COS-CMC可能是宫颈癌临床治疗的候选药物。促使内体逃逸并将药物输送到HeLa核中。用200μg/ mL的MSNs-COS-CMC处理后,炎性细胞因子IL-6和TNF-α水平分别降低了70%和80%。DOX @ MSNs-COS-CMC的肿瘤抑制作用比游离DOX高0.4倍,从而减轻了HeLa异种移植肿瘤模型的心脏毒性和炎症。可逆电荷的DOX @ MSNs-COS-CMC可能是宫颈癌临床治疗的候选药物。



























 京公网安备 11010802027423号
京公网安备 11010802027423号