当前位置:
X-MOL 学术
›
Cryst. Growth Des.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Influence of C–H/X (X = S, Cl, N, Pt/Pd) Interactions on the Molecular and Crystal Structures of Pt(II) and Pd(II) Complexes with Thiomorpholine-4-carbonitrile: Crystallographic, Thermal, and DFT Study
Crystal Growth & Design ( IF 3.8 ) Pub Date : 2020-03-30 , DOI: 10.1021/acs.cgd.9b01661 Predrag Ristić 1 , Vladimir Blagojević 2 , Goran Janjić 3 , Marko Rodić 4 , Predrag Vulić 5 , Morgan Donnard 6 , Mihaela Gulea 7 , Agnieszka Chylewska 8 , Mariusz Makowski 8 , Tamara Todorović 1 , Nenad Filipović 9
Crystal Growth & Design ( IF 3.8 ) Pub Date : 2020-03-30 , DOI: 10.1021/acs.cgd.9b01661 Predrag Ristić 1 , Vladimir Blagojević 2 , Goran Janjić 3 , Marko Rodić 4 , Predrag Vulić 5 , Morgan Donnard 6 , Mihaela Gulea 7 , Agnieszka Chylewska 8 , Mariusz Makowski 8 , Tamara Todorović 1 , Nenad Filipović 9
Affiliation
|
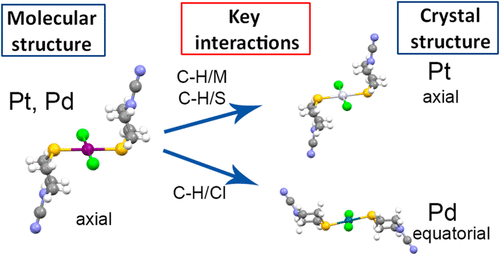
Pt(II) and Pd(II) complexes (1 and 2, respectively) with thiomorpholine-4-carbonitrile (TM-CN), an N-substituted thiomorpholine derivative, were synthesized from tetrachlorido precursors in water. Structural analysis has shown that 1 represents the first monomeric metal complex with this ligand type with an axial M–S bond with respect to the TM-CN ring chair conformation, while in 2 a typical equatorial M–S bond position with respect to the ring chair conformation was observed. A detailed DFT investigation revealed that axial conformers are more stable for molecular forms of both metals, while intermolecular interactions in the crystals stabilize the axial conformer for Pt(II) and the equatorial conformer for Pd(II). The magnitude of this stabilization in the case of 2 is large enough to change the most stable axial conformer in the molecular form to the equatorial conformer in the crystal. Further investigation of the strength of individual intermolecular interactions revealed significant differences of some interactions between the two structures. The likely cause of the difference in the crystal structures of experimentally obtained complexes is the fact that 1 and 2 exhibit different dominant interactions: C–H/M and C–H/S are more dominant in 1 and C–H/Cl interactions are more dominant in 2. In addition, DFT calculations have shown that while the axial position of the Pt–S bond with respect to the ring chair conformation results in a significantly shorter C–H/Pt interaction distance than that in the hypothetical equatorial conformer, there is very little difference in C–H/Pd interaction distances in conformers with axial and equatorial positions of Pd–S bond with respect to the ring chair conformation.
中文翻译:

C–H / X(X = S,Cl,N,Pt / Pd)相互作用对带有硫吗啉-4-腈的Pt(II)和Pd(II)配合物的分子和晶体结构的影响:晶体学,热学和DFT研究
从水中的四氯化物前体合成了Pt(II)和Pd(II)配合物(分别为1和2)与N-取代的硫代吗啉衍生物-巯基吗啉-4-腈(TM-CN)。结构分析表明,1表示具有该配体类型的第一个单体金属配合物,相对于TM-CN环椅构象具有轴向M–S键,而2表示观察到相对于环椅构型的典型赤道M–S键位置。DFT的详细研究表明,轴向构象异构体对于两种金属的分子形式都更稳定,而晶体中的分子间相互作用则稳定了Pt(II)的轴向构象和Pd(II)的赤道构象。在2的情况下,这种稳定作用的大小足够大,足以将分子形式最稳定的轴向构象异构体转变为晶体中的赤道构象异构体。进一步研究单个分子间相互作用的强度,发现两种结构之间某些相互作用存在显着差异。实验获得的配合物晶体结构差异的可能原因是:1和2表现出不同的显性相互作用:C–H / M和C–H / S在1中更为显性,而C–H / Cl相互作用在2中更为显性。此外,DFT计算表明,尽管Pt–S键相对于环椅构象的轴向位置导致的C–H / Pt相互作用距离比假设的赤道构象者短得多,但差异很小相对于环椅构象,Pd-S键的轴向和赤道位置的构象异构体的C–H / Pd相互作用距离。
更新日期:2020-03-30
中文翻译:

C–H / X(X = S,Cl,N,Pt / Pd)相互作用对带有硫吗啉-4-腈的Pt(II)和Pd(II)配合物的分子和晶体结构的影响:晶体学,热学和DFT研究
从水中的四氯化物前体合成了Pt(II)和Pd(II)配合物(分别为1和2)与N-取代的硫代吗啉衍生物-巯基吗啉-4-腈(TM-CN)。结构分析表明,1表示具有该配体类型的第一个单体金属配合物,相对于TM-CN环椅构象具有轴向M–S键,而2表示观察到相对于环椅构型的典型赤道M–S键位置。DFT的详细研究表明,轴向构象异构体对于两种金属的分子形式都更稳定,而晶体中的分子间相互作用则稳定了Pt(II)的轴向构象和Pd(II)的赤道构象。在2的情况下,这种稳定作用的大小足够大,足以将分子形式最稳定的轴向构象异构体转变为晶体中的赤道构象异构体。进一步研究单个分子间相互作用的强度,发现两种结构之间某些相互作用存在显着差异。实验获得的配合物晶体结构差异的可能原因是:1和2表现出不同的显性相互作用:C–H / M和C–H / S在1中更为显性,而C–H / Cl相互作用在2中更为显性。此外,DFT计算表明,尽管Pt–S键相对于环椅构象的轴向位置导致的C–H / Pt相互作用距离比假设的赤道构象者短得多,但差异很小相对于环椅构象,Pd-S键的轴向和赤道位置的构象异构体的C–H / Pd相互作用距离。



























 京公网安备 11010802027423号
京公网安备 11010802027423号