当前位置:
X-MOL 学术
›
Chem. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Enzymes in a golden cage
Chemical Science ( IF 8.4 ) Pub Date : 2020-03-28 , DOI: 10.1039/c9sc05419g Yael Baruch-Shpigler 1, 2, 3, 4, 5 , David Avnir 1, 2, 3, 4, 5
Chemical Science ( IF 8.4 ) Pub Date : 2020-03-28 , DOI: 10.1039/c9sc05419g Yael Baruch-Shpigler 1, 2, 3, 4, 5 , David Avnir 1, 2, 3, 4, 5
Affiliation
|
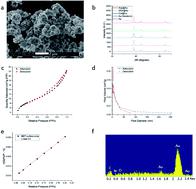
We describe a general method for the entrapment of enzymes within bulk metallic gold. This is a new approach for the immobilization of enzymes on metals, which is commonly carried out by 2D adsorption or covalent biding, that is, the enzyme is in contact with the metal at a specific contact zone of the enzyme, while most of the rest of it remains exposed to the environment. The 3D metallic encaging of the enzymes is quite different: the enzyme is in contact with the metallic cage walls all around it and is well protected inside. The porous nature of the metallic matrix enables substrate molecules to diffuse inside, reach the active site, and let product molecules diffuse out. The generality of the approach was proven by the successful entrapment of five enzymes representing different classes and different bio- and medical applications: L-asparaginase (Asp), collagenase, horseradish peroxidase (HRP), laccase and glucose oxidase (GOx). GOx–gold conjugates have been of particular interest in the literature. The main challenge we had to solve was how to keep the enzyme active in the process of gold-synthesis from its cation – this required careful tailoring of reaction conditions, which are detailed in the paper. The gold entrapped enzymes gain thermal stability and protectability against harsh conditions. For instance, we could keep Asp alive at the extreme pH of 13, which normally kills the enzyme instantly. The entrapped enzymes obey the Michaelis–Menten kinetics, and activation energies were determined. Good recyclability for eight cycles was found. Multi-enzymatic reactions by combinations of the off-the-shelf bioactive enzyme@gold powders are possible, as demonstrated for the classical detection of GOx activity with HRP. Detailed material characterization and proposed mechanisms for the 3D protectability of the enzymes are provided. The new enzyme immobilization method is of wide potential uses in medicine, biotechnology, bio-fuel cells and enzymatic (electro)sensing applications.
中文翻译:

金色笼子里的酶
我们描述了一种在大块金属金中捕获酶的一般方法。这是一种将酶固定在金属上的新方法,通常通过2D吸附或共价键合进行,即,酶在特定的酶接触区与金属接触,而其余大部分它仍然暴露在环境中。酶的3D金属包裹非常不同:酶与金属笼壁接触,并在内部受到良好保护。金属基质的多孔性质使底物分子能够在内部扩散,到达活性位点并让产物分子扩散出去。该方法的普遍性已通过成功捕获五种代表不同类别以及不同生物和医学应用的酶而得到证明:大号-天冬酰胺酶(Asp),胶原酶,辣根过氧化物酶(HRP),漆酶和葡萄糖氧化酶(GOx)。在文献中,GOx-金结合物特别受关注。我们必须解决的主要挑战是如何在阳离子的合成过程中保持酶的活性-这需要仔细调整反应条件,本文对此进行了详细介绍。包金的酶具有热稳定性和对恶劣条件的保护性。例如,我们可以使Asp在13的极端pH下保持活力,这通常会立即杀死该酶。截留的酶遵循米氏-门腾动力学,并确定了活化能。发现八个循环的良好可回收性。通过现成的生物活性酶@金粉的组合可以进行多种酶反应,如用HRP对GOx活性的经典检测所证明的。提供了详细的材料表征和酶3D保护性的拟议机制。这种新的酶固定方法在医学,生物技术,生物燃料电池和酶(电子)传感应用中具有广泛的潜在用途。
更新日期:2020-04-24
中文翻译:

金色笼子里的酶
我们描述了一种在大块金属金中捕获酶的一般方法。这是一种将酶固定在金属上的新方法,通常通过2D吸附或共价键合进行,即,酶在特定的酶接触区与金属接触,而其余大部分它仍然暴露在环境中。酶的3D金属包裹非常不同:酶与金属笼壁接触,并在内部受到良好保护。金属基质的多孔性质使底物分子能够在内部扩散,到达活性位点并让产物分子扩散出去。该方法的普遍性已通过成功捕获五种代表不同类别以及不同生物和医学应用的酶而得到证明:大号-天冬酰胺酶(Asp),胶原酶,辣根过氧化物酶(HRP),漆酶和葡萄糖氧化酶(GOx)。在文献中,GOx-金结合物特别受关注。我们必须解决的主要挑战是如何在阳离子的合成过程中保持酶的活性-这需要仔细调整反应条件,本文对此进行了详细介绍。包金的酶具有热稳定性和对恶劣条件的保护性。例如,我们可以使Asp在13的极端pH下保持活力,这通常会立即杀死该酶。截留的酶遵循米氏-门腾动力学,并确定了活化能。发现八个循环的良好可回收性。通过现成的生物活性酶@金粉的组合可以进行多种酶反应,如用HRP对GOx活性的经典检测所证明的。提供了详细的材料表征和酶3D保护性的拟议机制。这种新的酶固定方法在医学,生物技术,生物燃料电池和酶(电子)传感应用中具有广泛的潜在用途。



























 京公网安备 11010802027423号
京公网安备 11010802027423号