Stem Cell Research ( IF 1.2 ) Pub Date : 2020-03-28 , DOI: 10.1016/j.scr.2020.101770 Josef Večeřa 1 , Jiřina Procházková 2 , Veronika Šumberová 3 , Veronika Pánská 3 , Hana Paculová 2 , Martina Kohutková Lánová 3 , Jan Mašek 4 , Dáša Bohačiaková 5 , Emma Rachel Andersson 6 , Jiří Pacherník 3
|
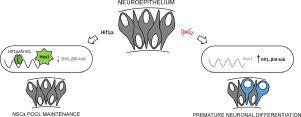
Embryonic neural stem cells (NSCs), comprising neuroepithelial and radial glial cells, are indispensable precursors of neurons and glia in the mammalian developing brain. Since the process of neurogenesis occurs in a hypoxic environment, the question arises of how NSCs deal with low oxygen tension and whether it affects their stemness. Genes from the hypoxia-inducible factors (HIF) family are well known factors governing cellular response to hypoxic conditions. In this study, we have discovered that the endogenous stabilization of hypoxia-inducible factor 1α (Hif1α) during neural induction is critical for the normal development of the NSCs pool by preventing its premature depletion and differentiation. The knock-out of the Hif1α gene in mESC-derived neurospheres led to a decrease in self-renewal of NSCs, paralleled by an increase in neuronal differentiation. Similarly, neuroepithelial cells differentiated in hypoxia exhibited accelerated neurogenesis soon after Hif1α knock-down. In both models, the loss of Hif1α was accompanied by an immediate drop in neural repressor Hes1 levels while changes in Notch signaling were not observed. We found that active Hif1α/Arnt1 transcription complex bound to the evolutionarily conserved site in Hes1 gene promoter in both neuroepithelial cells and neural tissue of E8.5 – 9.5 embryos. Taken together, these results emphasize the novel role of Hif1α in the regulation of early NSCs population through the activation of neural repressor Hes1, independently of Notch signaling.
中文翻译:

缺氧/Hif1α通过Hes1的激活阻止神经干细胞的神经元过早分化。
包括神经上皮和放射状神经胶质细胞的胚胎神经干细胞(NSC)是哺乳动物发育中的大脑中神经元和神经胶质必不可少的前体。由于神经发生过程发生在低氧环境中,因此产生了一个问题,即神经干细胞如何处理低氧张力以及它是否影响其干性。来自缺氧诱导因子(HIF)家族的基因是控制细胞对缺氧条件反应的众所周知的因子。在这项研究中,我们发现神经诱导过程中缺氧诱导因子1α(Hif1α)的内源性稳定通过防止NSC的过早耗竭和分化对于NSCs池的正常发育至关重要。Hif1α的敲除mESC衍生的神经球中的SNP基因导致NSC自我更新的减少,同时神经元分化的增加。类似地,在缺氧条件下分化的神经上皮细胞在Hif1α敲低后很快显示出加速的神经发生。在这两个模型中,Hif1α的丧失均伴随着神经阻遏物Hes1水平的立即下降,而未观察到Notch信号的变化。我们发现,活跃的Hif1α/ Arnt1转录复合体与神经上皮细胞和E8.5 – 9.5胚胎的神经组织中Hes1基因启动子中的进化保守位点结合。综上所述,这些结果强调了Hif1α通过激活神经阻遏物Hes1(独立于Notch信号传导)在早期NSC种群调控中的新作用。



























 京公网安备 11010802027423号
京公网安备 11010802027423号