Journal of Clinical Lipidology ( IF 4.4 ) Pub Date : 2020-03-28 , DOI: 10.1016/j.jacl.2020.03.001 Stephen Daniels 1 , Sonia Caprio 2 , Umesh Chaudhari 3 , Garen Manvelian 3 , Marie T Baccara-Dinet 4 , Aurelie Brunet 4 , Michel Scemama 5 , Virginie Loizeau 5 , Eric Bruckert 6
|
Background
Heterozygous familial hypercholesterolemia (HeFH) is a genetic disorder characterized by elevated levels of low-density lipoprotein cholesterol (LDL-C).
Objective
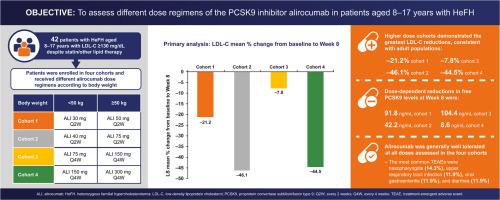
This phase 2 dose-finding study (NCT02890992) evaluated the efficacy, safety, and dose selection of proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitor alirocumab in pediatric HeFH patients.
Methods
HeFH patients (n = 42) who were aged 8–17 years, had body weight (BW) ≥25 kg, and had LDL-C ≥130 mg/dL despite optimal statin/other lipid-modifying therapies were enrolled in 4 cohorts according to BW: cohort #1: 30 mg (<50 kg) or 50 mg (≥50 kg) every 2 weeks (Q2W), #2: 40 mg (<50 kg) or 75 mg (≥50 kg) Q2W, #3: 75 mg (<50 kg) or 150 mg (≥50 kg) every 4 weeks (Q4W), #4: 150 mg (<50 kg) or 300 mg (≥50 kg) Q4W. Primary endpoint was LDL-C % change from baseline to week 8.
Results
Mean age was 12.4 years and 95% of patients were on a statin. Baseline LDL-C levels were 160.0–188.9 mg/dL and free PCSK9 was 186.4–201.7 ng/mL across the cohorts. At week 8, the higher dose cohorts (2 and 4) demonstrated the greatest reductions in LDL-C (–46% and –45%, respectively). Free PCSK9 levels were lowest at week 8 in cohorts 2 and 4 (42.2 ng/mL and 8.6 ng/mL, respectively). Adverse events were reported in 50–90% of patients across the cohorts, and 2 patients discontinued due to adverse events.
Conclusions
In pediatric HeFH patients, LDL-C reductions were greatest in the higher dose cohorts. Alirocumab was generally well tolerated at all doses.
中文翻译:

在患有杂合子家族性高胆固醇血症的儿科患者中使用 alirocumab 抑制 PCSK9:ODYSSEY KIDS 研究。
背景
杂合子家族性高胆固醇血症 (HeFH) 是一种遗传性疾病,其特征是低密度脂蛋白胆固醇 (LDL-C) 水平升高。
客观的
这项 2 期剂量探索研究 (NCT02890992) 评估了前蛋白转化酶枯草杆菌蛋白酶 / kexin 9 型 (PCSK9) 抑制剂 alirocumab 在儿童 HeFH 患者中的疗效、安全性和剂量选择。
方法
HeFH 患者(n = 42)年龄在 8-17 岁之间,体重 (BW) ≥ 25 kg,尽管接受了最佳他汀类药物/其他调脂疗法,但 LDL-C ≥ 130 mg/dL 被纳入 4 个队列。至 BW:队列 #1:每 2 周(Q2W)30 毫克(<50 公斤)或 50 毫克(≥50 公斤),#2:40 毫克(<50 公斤)或 75 毫克(≥50 公斤)Q2W,# 3:每 4 周(Q4W)75 毫克(<50 公斤)或 150 毫克(≥50 公斤),#4:150 毫克(<50 公斤)或 300 毫克(≥50 公斤)Q4W。主要终点是从基线到第 8 周的 LDL-C 百分比变化。
结果
平均年龄为 12.4 岁,95% 的患者服用他汀类药物。整个队列的基线 LDL-C 水平为 160.0-188.9 mg/dL,游离 PCSK9 为 186.4-201.7 ng/mL。在第 8 周,较高剂量组(2 和 4)的 LDL-C 降低幅度最大(分别为 –46% 和 –45%)。在第 8 周,第 2 和第 4 组的游离 PCSK9 水平最低(分别为 42.2 ng/mL 和 8.6 ng/mL)。整个队列中 50-90% 的患者报告了不良事件,2 名患者因不良事件而停药。
结论
在儿科 HeFH 患者中,高剂量组的 LDL-C 降低幅度最大。Alirocumab 在所有剂量下的耐受性普遍良好。



























 京公网安备 11010802027423号
京公网安备 11010802027423号