当前位置:
X-MOL 学术
›
Energy Convers. Manag.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
A highly competitive system for CO methanation over an active metal-free fibrous silica mordenite via in-situ ESR and FTIR studies
Energy Conversion and Management ( IF 10.4 ) Pub Date : 2020-05-01 , DOI: 10.1016/j.enconman.2020.112754 I. Hussain , A.A. Jalil , N.A.A. Fatah , M.Y.S. Hamid , M. Ibrahim , M.A.A. Aziz , H.D. Setiabudi
Energy Conversion and Management ( IF 10.4 ) Pub Date : 2020-05-01 , DOI: 10.1016/j.enconman.2020.112754 I. Hussain , A.A. Jalil , N.A.A. Fatah , M.Y.S. Hamid , M. Ibrahim , M.A.A. Aziz , H.D. Setiabudi
|
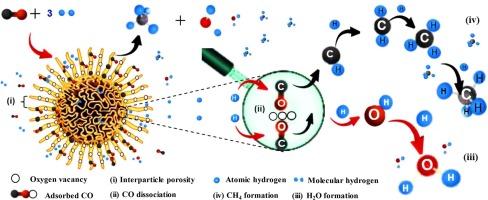
Abstract Catalytic methanation of carbon monoxide (CO) offers a sustainable and attractive way to produce the synthetic natural gas (SNG), which can be a substitute for fossil fuels (coal, petroleum and natural gas) towards a low carbon future. This study focuses on CO methanation over a modified mordenite (FSMOR), which was synthesized through a microemulsion method. The Physico-chemical properties of the synthesized FSMOR were examined by field emission scanning electron microscope (FESEM), X-ray diffraction (XRD), transmission electron microscopy (TEM), N2 adsorption-desorption isotherms, and electron spin resonance (ESR). The FSMOR showed a unique fibrous morphology, which has improved the CO conversion (73%), CH4 selectivity (71%) and rate of formation (0.0491 µmol-CH4/m2s) remarkably due to enhancement in BET surface area, oxygen vacancies, and basicity. The FSMOR expressed high thermal stability and low carbon deposition compared to MOR, which was confirmed by thermogravimetric analysis (TGA), Raman and TEM observations. Besides, the in-situ ESR and FTIR observations proposed that the oxygen vacancies played a vital role to adsorb and activate the CO and H2 molecules via linear adsorbed CO* as intermediates, which dissociated into adsorbed C* to form methane by hydrogenation. This study may open up new opportunities for metal-free heterogeneous catalysis systems to enhance the catalytic CO methanation to produce SNG.
中文翻译:

通过原位 ESR 和 FTIR 研究在活性无金属纤维状二氧化硅丝光沸石上进行 CO 甲烷化的极具竞争力的系统
摘要 一氧化碳 (CO) 的催化甲烷化为生产合成天然气 (SNG) 提供了一种可持续且有吸引力的方法,可以替代化石燃料(煤、石油和天然气),迈向低碳未来。本研究侧重于通过微乳液法合成的改性丝光沸石 (FSMOR) 上的 CO 甲烷化。通过场发射扫描电子显微镜 (FESEM)、X 射线衍射 (XRD)、透射电子显微镜 (TEM)、N2 吸附-解吸等温线和电子自旋共振 (ESR) 检查合成的 FSMOR 的物理化学性质。FSMOR 显示出独特的纤维形态,由于 BET 表面积、氧空位、氧空位的增强,显着提高了 CO 转化率 (73%)、CH4 选择性 (71%) 和形成速率 (0.0491 µmol-CH4/m2s)。和碱性。与 MOR 相比,FSMOR 表现出高热稳定性和低碳沉积,这通过热重分析 (TGA)、拉曼和 TEM 观察得到证实。此外,原位 ESR 和 FTIR 观察表明,氧空位通过线性吸附的 CO* 作为中间体,在吸附和活化 CO 和 H2 分子方面起着至关重要的作用,CO* 作为中间体,通过加氢分解成吸附的 C* 形成甲烷。这项研究可能为无金属多相催化系统开辟新的机会,以增强催化 CO 甲烷化以生产 SNG。原位 ESR 和 FTIR 观察表明,氧空位通过线性吸附的 CO* 作为中间体,在吸附和活化 CO 和 H2 分子方面起着至关重要的作用,CO* 作为中间体,通过加氢分解成吸附的 C* 形成甲烷。这项研究可能为无金属多相催化系统开辟新的机会,以增强催化 CO 甲烷化以生产 SNG。原位 ESR 和 FTIR 观察表明,氧空位通过线性吸附的 CO* 作为中间体,在吸附和活化 CO 和 H2 分子方面起着至关重要的作用,CO* 作为中间体,通过加氢分解成吸附的 C* 形成甲烷。这项研究可能为无金属多相催化系统开辟新的机会,以增强催化 CO 甲烷化以生产 SNG。
更新日期:2020-05-01
中文翻译:

通过原位 ESR 和 FTIR 研究在活性无金属纤维状二氧化硅丝光沸石上进行 CO 甲烷化的极具竞争力的系统
摘要 一氧化碳 (CO) 的催化甲烷化为生产合成天然气 (SNG) 提供了一种可持续且有吸引力的方法,可以替代化石燃料(煤、石油和天然气),迈向低碳未来。本研究侧重于通过微乳液法合成的改性丝光沸石 (FSMOR) 上的 CO 甲烷化。通过场发射扫描电子显微镜 (FESEM)、X 射线衍射 (XRD)、透射电子显微镜 (TEM)、N2 吸附-解吸等温线和电子自旋共振 (ESR) 检查合成的 FSMOR 的物理化学性质。FSMOR 显示出独特的纤维形态,由于 BET 表面积、氧空位、氧空位的增强,显着提高了 CO 转化率 (73%)、CH4 选择性 (71%) 和形成速率 (0.0491 µmol-CH4/m2s)。和碱性。与 MOR 相比,FSMOR 表现出高热稳定性和低碳沉积,这通过热重分析 (TGA)、拉曼和 TEM 观察得到证实。此外,原位 ESR 和 FTIR 观察表明,氧空位通过线性吸附的 CO* 作为中间体,在吸附和活化 CO 和 H2 分子方面起着至关重要的作用,CO* 作为中间体,通过加氢分解成吸附的 C* 形成甲烷。这项研究可能为无金属多相催化系统开辟新的机会,以增强催化 CO 甲烷化以生产 SNG。原位 ESR 和 FTIR 观察表明,氧空位通过线性吸附的 CO* 作为中间体,在吸附和活化 CO 和 H2 分子方面起着至关重要的作用,CO* 作为中间体,通过加氢分解成吸附的 C* 形成甲烷。这项研究可能为无金属多相催化系统开辟新的机会,以增强催化 CO 甲烷化以生产 SNG。原位 ESR 和 FTIR 观察表明,氧空位通过线性吸附的 CO* 作为中间体,在吸附和活化 CO 和 H2 分子方面起着至关重要的作用,CO* 作为中间体,通过加氢分解成吸附的 C* 形成甲烷。这项研究可能为无金属多相催化系统开辟新的机会,以增强催化 CO 甲烷化以生产 SNG。



























 京公网安备 11010802027423号
京公网安备 11010802027423号