Journal of Photochemistry and Photobiology A: Chemistry ( IF 4.3 ) Pub Date : 2020-03-27 , DOI: 10.1016/j.jphotochem.2020.112505 Ma. Elena Manríquez , Martín Trejo Valdez , Andrea Sánchez Pliego , Laura V. Castro , Emma Ortiz-Islas
|
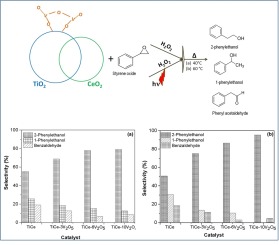
This work reports the preparation of the TiO2-CeO2 (TiCe) catalytic support of V2O5 catalysts, which was tested in the oxidation process of styrene oxide via chemical and photochemical methods. The TiCe-V2O5 catalytic support was prepared by the co-precipitated method from the individual metal oxides, varying the amount of vanadium oxide by 3, 6, and 10 % mol with respect to the support. The obtained catalysts were characterized by different spectroscopies, as well as by the N2 adsorption-desorption technique. The catalytic reaction test was carried out in the liquid phase during 120 min with/without ultraviolet light irradiation at 50 °C. There was no V2O5 effect on the surface area, pore volume, and pore diameter since all catalysts had similar textural values. In all samples the structures identified by X-ray diffraction were the Anatase phase and CeO2 in the cubic phase. XPS results revealed the formation of surface carbonate species, which were also identified by infrared spectroscopy. The conversion rate was better when employing ultraviolet light, and the rate increased as the V2O5 amount rose. The main reaction products were 2-phenylethanol and 1-phenylethanol. However, a low amount of benzaldehyde was detected. The selectivity to the desirable product (2-phenylethanol) increased when the reaction was irradiated with UV light and the catalyst contained a higher amount of vanadium. It was observed that the effect of UV radiation on the electric mobility produces an acceleration of the reaction to 2-phenylethanol, avoiding the 1-phenylethanol formation. The bandgap value decreased as the vanadium oxide amount increased, boosting the electric mobility.
中文翻译:

使用TiO 2 -CeO 2 -V 2 O 5催化剂通过化学和光化学方法氧化苯乙烯氧化物
这项工作报告了V 2 O 5催化剂的TiO 2 -CeO 2(TiCe)催化载体的制备,该方法已通过化学和光化学方法在苯乙烯氧化物的氧化过程中进行了测试。TiCe-V 2 O 5催化载体是通过共沉淀法由各种金属氧化物制备的,其中钒氧化物的量相对于载体为3%,6%和10%mol。所获得的催化剂通过不同的光谱学以及N 2吸附-解吸技术进行表征。催化反应测试是在50分钟内在有/无紫外线照射下于120分钟内在液相中进行的。没有V2 O 5对表面积,孔体积和孔径的影响,因为所有催化剂具有相似的质地值。在所有样品中,通过X射线衍射鉴定的结构为锐钛矿相和立方相中的CeO 2。XPS结果揭示了表面碳酸盐物质的形成,也可以通过红外光谱法鉴定。当使用紫外线时,转化率更好,并且随着V 2 O 5的增加,转化率增加。金额上升。主要反应产物是2-苯乙醇和1-苯乙醇。但是,检测到少量的苯甲醛。当用UV光照射反应并且催化剂包含更高量的钒时,对所需产物(2-苯基乙醇)的选择性增加。观察到紫外线辐射对电迁移率的影响使向2-苯基乙醇的反应加速,避免了1-苯基乙醇的形成。带隙值随着氧化钒量的增加而降低,从而提高了电迁移率。



























 京公网安备 11010802027423号
京公网安备 11010802027423号