当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
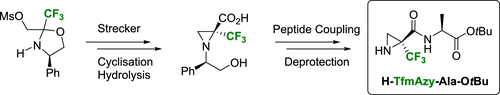
Enantiopure α-Trifluoromethylated Aziridine-2-carboxylic Acid (α-TfmAzy): Synthesis and Peptide Coupling.
Organic Letters ( IF 5.2 ) Pub Date : 2020-03-27 , DOI: 10.1021/acs.orglett.0c00645 Oussema Ouerfelli 1, 2, 3 , Julien Simon 1, 2 , Evelyne Chelain 1, 2 , Julien Pytkowicz 1, 2 , Rafâa Besbes 3 , Thierry Brigaud 1, 2
Organic Letters ( IF 5.2 ) Pub Date : 2020-03-27 , DOI: 10.1021/acs.orglett.0c00645 Oussema Ouerfelli 1, 2, 3 , Julien Simon 1, 2 , Evelyne Chelain 1, 2 , Julien Pytkowicz 1, 2 , Rafâa Besbes 3 , Thierry Brigaud 1, 2
Affiliation
|
A straightforward synthesis of enantiopure α-trifluoromethyl aziridine-2-carboxylic acid (α-TfmAzy) is reported from a trifluoropyruvate derived enantiopure oxazolidine. A key Strecker-type synthetic step and a late cyanide basic hydrolysis gave the target compounds in six steps and 41% yield. A final peptide coupling was performed to demonstrate the usefulness of this highly constrained fluorinated unnatural amino acid.
中文翻译:

对映纯α-三氟甲基化氮丙啶-2-羧酸(α-TfmAzy):合成和肽偶联。
据报道,由三氟丙酮酸衍生的对映体纯的恶唑烷可直接合成对映体纯的α-三氟甲基氮丙啶-2-羧酸(α-TfmAzy)。关键的Strecker型合成步骤和后期氰化物碱性水解反应可分六步完成,目标化合物收率为41%。进行了最终的肽偶联以证明这种高度受限的氟化非天然氨基酸的有用性。
更新日期:2020-04-24
中文翻译:

对映纯α-三氟甲基化氮丙啶-2-羧酸(α-TfmAzy):合成和肽偶联。
据报道,由三氟丙酮酸衍生的对映体纯的恶唑烷可直接合成对映体纯的α-三氟甲基氮丙啶-2-羧酸(α-TfmAzy)。关键的Strecker型合成步骤和后期氰化物碱性水解反应可分六步完成,目标化合物收率为41%。进行了最终的肽偶联以证明这种高度受限的氟化非天然氨基酸的有用性。



























 京公网安备 11010802027423号
京公网安备 11010802027423号