当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
An Asymmetric Suzuki-Miyaura Approach to Prostaglandins: Synthesis of Tafluprost.
Organic Letters ( IF 5.2 ) Pub Date : 2020-03-27 , DOI: 10.1021/acs.orglett.0c00745 Roman Kučera 1 , F Wieland Goetzke 1 , Stephen P Fletcher 1
Organic Letters ( IF 5.2 ) Pub Date : 2020-03-27 , DOI: 10.1021/acs.orglett.0c00745 Roman Kučera 1 , F Wieland Goetzke 1 , Stephen P Fletcher 1
Affiliation
|
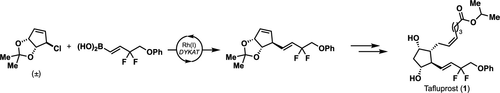
We report the catalytic asymmetric synthesis of Tafluprost (1), a prostaglandin analogue. This synthesis demonstrates a new approach to prostaglandins involving symmetrization and desymmetrization of a racemic precursor to control the absolute and relative stereochemistry of the cyclopentyl core. Key steps include a diastereo- and enantioselective Rh-catalyzed Suzuki–Miyaura reaction of a racemic bicyclic allyl chloride and an alkenyl boronic acid and a regio- and diastereoselective Pd-catalyzed Tsuji–Trost reaction with an enolate surrogate.
中文翻译:

前列腺素的不对称Suzuki-Miyaura方法:塔氟前列素的合成。
我们报告了前列腺素类似物Tafluprost(1)的催化不对称合成。该合成证明了前列腺素的新方法,涉及外消旋前体的对称和去对称,以控制环戊基核心的绝对和相对立体化学。关键步骤包括外消旋双环烯丙基氯和烯基硼酸的非对映和对映选择性Rh催化的Suzuki-Miyaura反应,以及区域和非对映选择性Pd催化的Tsuji-Trost反应和一个烯醇式替代物。
更新日期:2020-04-24
中文翻译:

前列腺素的不对称Suzuki-Miyaura方法:塔氟前列素的合成。
我们报告了前列腺素类似物Tafluprost(1)的催化不对称合成。该合成证明了前列腺素的新方法,涉及外消旋前体的对称和去对称,以控制环戊基核心的绝对和相对立体化学。关键步骤包括外消旋双环烯丙基氯和烯基硼酸的非对映和对映选择性Rh催化的Suzuki-Miyaura反应,以及区域和非对映选择性Pd催化的Tsuji-Trost反应和一个烯醇式替代物。


























 京公网安备 11010802027423号
京公网安备 11010802027423号