当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Catalytic Enantioselective Total Synthesis of (+)-Lycoperdic Acid.
Organic Letters ( IF 5.2 ) Pub Date : 2020-03-27 , DOI: 10.1021/acs.orglett.0c00772 Sami Kortet 1 , Aurélie Claraz 1 , Petri M Pihko 1
Organic Letters ( IF 5.2 ) Pub Date : 2020-03-27 , DOI: 10.1021/acs.orglett.0c00772 Sami Kortet 1 , Aurélie Claraz 1 , Petri M Pihko 1
Affiliation
|
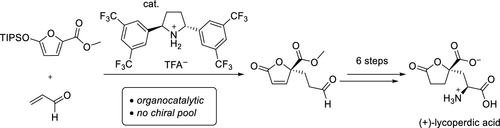
A concise enantio- and stereocontrolled synthesis of (+)-lycoperdic acid is presented. The stereochemical control is based on iminium-catalyzed Mukaiyama–Michael reaction and enamine-catalyzed organocatalytic α-chlorination steps. The amino group was introduced by azide displacement, affording the final stereochemistry of (+)-lycoperdic acid. Penultimate hydrogenation and hydrolysis afforded pure (+)-lycoperdic acid in seven steps from a known silyloxyfuran.
中文翻译:

(+)-Lycoperdic acid的催化对映选择性全合成。
简明的对映体和立体控制合成(+)-lyperperdic酸。立体化学控制基于亚胺基催化的Mukaiyama-Michael反应和烯胺催化的有机催化α-氯化步骤。通过叠氮化物置换引入氨基,从而提供(+)-乙过二酸的最终立体化学。倒数第二次氢化和水解从已知的甲硅烷氧基呋喃分七步得到纯的(+)-乙二酸。
更新日期:2020-04-24
中文翻译:

(+)-Lycoperdic acid的催化对映选择性全合成。
简明的对映体和立体控制合成(+)-lyperperdic酸。立体化学控制基于亚胺基催化的Mukaiyama-Michael反应和烯胺催化的有机催化α-氯化步骤。通过叠氮化物置换引入氨基,从而提供(+)-乙过二酸的最终立体化学。倒数第二次氢化和水解从已知的甲硅烷氧基呋喃分七步得到纯的(+)-乙二酸。



























 京公网安备 11010802027423号
京公网安备 11010802027423号