当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Highly Regio- and Enantioselective Hydrogenation of Conjugated α-Substituted Dienoic Acids.
Organic Letters ( IF 5.2 ) Pub Date : 2020-03-27 , DOI: 10.1021/acs.orglett.0c00915 Xian Liu 1 , Song Liu 2 , Quanjun Wang 1 , Gang Zhou 1 , Lin Yao 1 , Qin Ouyang 3 , Ru Jiang 1 , Yu Lan 2, 4 , Weiping Chen 1
Organic Letters ( IF 5.2 ) Pub Date : 2020-03-27 , DOI: 10.1021/acs.orglett.0c00915 Xian Liu 1 , Song Liu 2 , Quanjun Wang 1 , Gang Zhou 1 , Lin Yao 1 , Qin Ouyang 3 , Ru Jiang 1 , Yu Lan 2, 4 , Weiping Chen 1
Affiliation
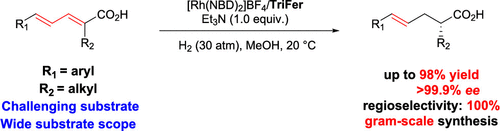
|
Highly regio- and enantioselective hydrogenation of conjugated α-substituted dienoic acids was realized for the first time using Trifer–Rh complex, providing a straightforward method for the synthesis of chiral α-substituted γ,δ-unsaturated acids. DFT calculations revealed N+H–O hydrogen bonding interaction is formed to stabilize the transition state and the coordination of 4,5-double bond to Rh(III) center would facilitate the reductive elimination process. This hydrogenation provided a gram-scale synthesis of the precursor of sacubitril.
中文翻译:

共轭α-取代的二烯酸的高度区域和对映选择性加氢。
使用Trifer-Rh配合物首次实现了对共轭α-取代二烯酸的高度区域和对映选择性加氢,为合成手性α-取代的γ,δ-不饱和酸提供了一种简单的方法。DFT计算表明,形成N + H–O氢键相互作用可稳定过渡态,并且4,5-双键与Rh(III)中心的配位将促进还原消除过程。该氢化提供了沙比特利前体的克级合成。
更新日期:2020-04-24
中文翻译:

共轭α-取代的二烯酸的高度区域和对映选择性加氢。
使用Trifer-Rh配合物首次实现了对共轭α-取代二烯酸的高度区域和对映选择性加氢,为合成手性α-取代的γ,δ-不饱和酸提供了一种简单的方法。DFT计算表明,形成N + H–O氢键相互作用可稳定过渡态,并且4,5-双键与Rh(III)中心的配位将促进还原消除过程。该氢化提供了沙比特利前体的克级合成。



























 京公网安备 11010802027423号
京公网安备 11010802027423号