当前位置:
X-MOL 学术
›
Chem. Commun.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Ring-opening iodination and bromination of unstrained cycloalkanols through β-scission of alkoxy radicals.
Chemical Communications ( IF 4.9 ) Pub Date : 2020-04-02 , DOI: 10.1039/d0cc01720e Jiang-Ling Shi 1 , Yuankai Wang , Zixuan Wang , Bowen Dou , Jianbo Wang
Chemical Communications ( IF 4.9 ) Pub Date : 2020-04-02 , DOI: 10.1039/d0cc01720e Jiang-Ling Shi 1 , Yuankai Wang , Zixuan Wang , Bowen Dou , Jianbo Wang
Affiliation
|
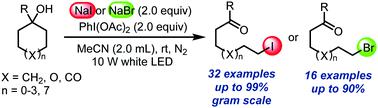
Ring-opening iodination or bromination of unstrained cycloalkanols with NaI or NaBr and PhI(OAc)2 under visible light irradiation is developed. In this protocol the concentration of I2 is modulated through the generation of triiodide (I3-), thus significantly avoiding undesired side reactions. The reaction is under mild conditions and has a wide substrate scope, thus providing a practically useful method for accessing ω-iodo or ω-bromoketones.
中文翻译:

通过β-烷氧基自由基的分裂,使未应变的环烷醇开环碘化和溴化。
在可见光照射下,开发了未应变的环烷醇与NaI或NaBr和PhI(OAc)2的开环碘化或溴化反应。在该方案中,通过生成三碘化物(I3-)来调节I2的浓度,从而显着避免了不良的副反应。该反应在温和的条件下进行并且具有广泛的底物范围,因此提供了用于获得ω-碘或ω-溴代酮的实用方法。
更新日期:2020-03-27
中文翻译:

通过β-烷氧基自由基的分裂,使未应变的环烷醇开环碘化和溴化。
在可见光照射下,开发了未应变的环烷醇与NaI或NaBr和PhI(OAc)2的开环碘化或溴化反应。在该方案中,通过生成三碘化物(I3-)来调节I2的浓度,从而显着避免了不良的副反应。该反应在温和的条件下进行并且具有广泛的底物范围,因此提供了用于获得ω-碘或ω-溴代酮的实用方法。



























 京公网安备 11010802027423号
京公网安备 11010802027423号