当前位置:
X-MOL 学术
›
Signal Transduct. Target Ther.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
The Agpat4/LPA axis in colorectal cancer cells regulates antitumor responses via p38/p65 signaling in macrophages.
Signal Transduction and Targeted Therapy ( IF 39.3 ) Pub Date : 2020-03-27 , DOI: 10.1038/s41392-020-0117-y Dapeng Zhang 1, 2 , Rongchen Shi 2 , Wei Xiang 2 , Xia Kang 2 , Bo Tang 3 , Chuan Li 3 , Linfeng Gao 3 , Xuan Zhang 4 , Lili Zhang 5 , Rongyang Dai 1 , Hongming Miao 1, 2
Signal Transduction and Targeted Therapy ( IF 39.3 ) Pub Date : 2020-03-27 , DOI: 10.1038/s41392-020-0117-y Dapeng Zhang 1, 2 , Rongchen Shi 2 , Wei Xiang 2 , Xia Kang 2 , Bo Tang 3 , Chuan Li 3 , Linfeng Gao 3 , Xuan Zhang 4 , Lili Zhang 5 , Rongyang Dai 1 , Hongming Miao 1, 2
Affiliation
|
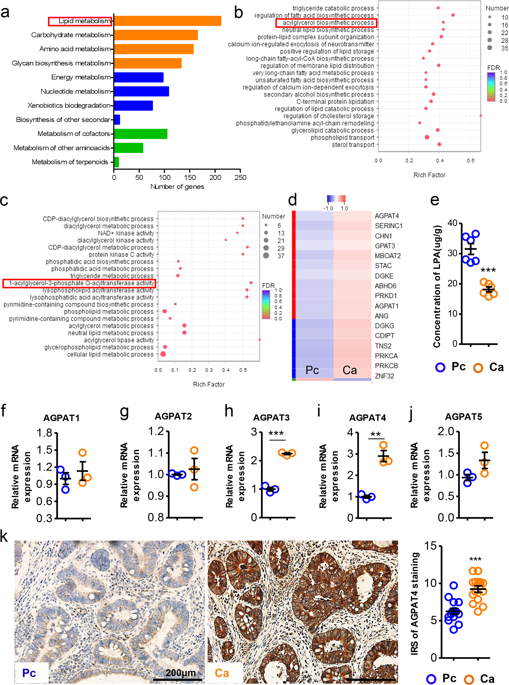
Lipid metabolic reprogramming plays an essential role in regulating the progression of colorectal cancer (CRC). However, the effect of lysophosphatidic acid (LPA) metabolism on CRC development is incompletely characterized. Here, we compared the mRNA levels of human CRC tissues to those of paracarcinoma tissues and focused on the notably enriched LPA metabolic pathways. We identified and verified that 1-acylglycerol-3-phosphate O-acyltransferase 4 (Agpat4) was aberrantly expressed in CRC tissues and predicted poor survival in CRC patients. Manipulating Agpat4 expression in CRC cells did not affect the growth or migration of CRC cells in vitro, whereas Agpat4 silencing suppressed CRC cell growth in subcutaneous and peritoneal xenograft models. Mechanistically, Agpat4 silencing-induced LPA release from CRC cells and polarized macrophages to an M1-like phenotype through LPA receptors 1 and 3. This M1 activation, characterized by elevated p38/p65 signaling and increased proinflammatory cytokines, promoted the infiltration and activation of CD4+ and CD8+ T cells in the tumor microenvironment. Modulation of the Agpat4/LPA/p38/p65 axis regulated macrophage polarization, T-cell activity and CRC progression. Notably, combined therapy with LPA and regular chemotherapy drugs synergistically suppressed CRC development. Taken together, our results showed that the Agpat4/LPA axis in CRC cells regulated p38/p65 signaling-dependent macrophage polarization, T-cell activation, and CRC progression. The Agpat4/LPA/p38/p65 axis might represent a potential target for therapy in the clinic.
中文翻译:

结直肠癌细胞中的 Agpat4/LPA 轴通过巨噬细胞中的 p38/p65 信号传导调节抗肿瘤反应。
脂质代谢重编程在调节结直肠癌 (CRC) 的进展中起着至关重要的作用。然而,溶血磷脂酸 (LPA) 代谢对 CRC 发展的影响尚不完全清楚。在这里,我们将人类 CRC 组织的 mRNA 水平与癌旁组织的 mRNA 水平进行了比较,并重点关注显着富集的 LPA 代谢途径。我们鉴定并验证了 1-酰基甘油-3-磷酸 O-酰基转移酶 4 (Agpat4) 在 CRC 组织中异常表达,并预测 CRC 患者的生存期较差。操纵 CRC 细胞中的 Agpat4 表达不影响体外 CRC 细胞的生长或迁移,而 Agpat4 沉默抑制了皮下和腹膜异种移植模型中的 CRC 细胞生长。从机制上讲,Agpat4 沉默诱导的 LPA 从 CRC 细胞和极化巨噬细胞通过 LPA 受体 1 和 3 释放为 M1 样表型。 这种 M1 激活,以 p38/p65 信号传导升高和促炎细胞因子增加为特征,促进了 CD4+ 和 CD8+ 的浸润和激活肿瘤微环境中的 T 细胞。Agpat4/LPA/p38/p65 轴的调节可调节巨噬细胞极化、T 细胞活性和 CRC 进展。值得注意的是,LPA 和常规化疗药物的联合治疗协同抑制了 CRC 的发展。总之,我们的结果表明 CRC 细胞中的 Agpat4/LPA 轴调节 p38/p65 信号依赖性巨噬细胞极化、T 细胞活化和 CRC 进展。Agpat4/LPA/p38/p65 轴可能代表临床治疗的潜在目标。
更新日期:2020-03-27
中文翻译:

结直肠癌细胞中的 Agpat4/LPA 轴通过巨噬细胞中的 p38/p65 信号传导调节抗肿瘤反应。
脂质代谢重编程在调节结直肠癌 (CRC) 的进展中起着至关重要的作用。然而,溶血磷脂酸 (LPA) 代谢对 CRC 发展的影响尚不完全清楚。在这里,我们将人类 CRC 组织的 mRNA 水平与癌旁组织的 mRNA 水平进行了比较,并重点关注显着富集的 LPA 代谢途径。我们鉴定并验证了 1-酰基甘油-3-磷酸 O-酰基转移酶 4 (Agpat4) 在 CRC 组织中异常表达,并预测 CRC 患者的生存期较差。操纵 CRC 细胞中的 Agpat4 表达不影响体外 CRC 细胞的生长或迁移,而 Agpat4 沉默抑制了皮下和腹膜异种移植模型中的 CRC 细胞生长。从机制上讲,Agpat4 沉默诱导的 LPA 从 CRC 细胞和极化巨噬细胞通过 LPA 受体 1 和 3 释放为 M1 样表型。 这种 M1 激活,以 p38/p65 信号传导升高和促炎细胞因子增加为特征,促进了 CD4+ 和 CD8+ 的浸润和激活肿瘤微环境中的 T 细胞。Agpat4/LPA/p38/p65 轴的调节可调节巨噬细胞极化、T 细胞活性和 CRC 进展。值得注意的是,LPA 和常规化疗药物的联合治疗协同抑制了 CRC 的发展。总之,我们的结果表明 CRC 细胞中的 Agpat4/LPA 轴调节 p38/p65 信号依赖性巨噬细胞极化、T 细胞活化和 CRC 进展。Agpat4/LPA/p38/p65 轴可能代表临床治疗的潜在目标。


























 京公网安备 11010802027423号
京公网安备 11010802027423号