Communications Chemistry ( IF 5.9 ) Pub Date : 2020-03-26 , DOI: 10.1038/s42004-020-0285-2 Kun Wang 1, 2 , Chang Xu 1 , Dan Li 1 , Longjiu Cheng 1, 2
|
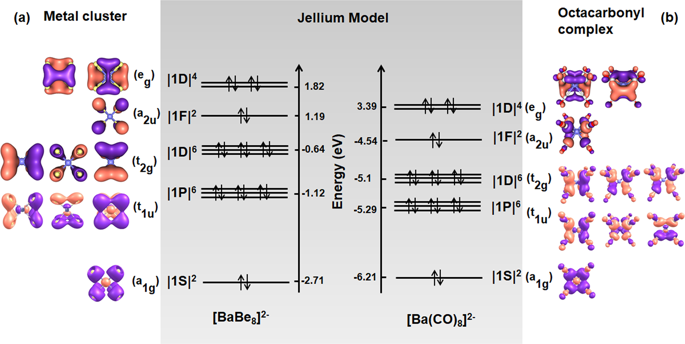
The recently reported octacarbonyl metal complexes M(CO)8 (M = Ca, Sr, Ba) feature interesting bonding structures. In these compounds, the bond order is 7, while accommodating 8 lone pairs of ligands in forming octa-coordinated complexes or ions. Here, by comparing [Ba(CO)8]2− and metal clusters of [BaBe8]2− analogically, we demonstrate that the Jellium model can not only be applied on metal clusters, but is also a useful tool to understand the electronic structures of [M(CO)8]q (M, q = Ca, 2−; Sc, 1−; Ti, 0; V, 1+; Cr, 2+; Ba, 2−). By applying the Jellium model, we find that a 20-e model with the configuration |1S2|1P6|1D10|1F2| is an appropriate description of the valence bonding structures of M(CO)8 species, where each coordinative bond contains 7/8ths of the bonding orbitals and 1/8th non-bonding orbitals.
中文翻译:

将 Jellium 模型应用于八羰基金属络合物
最近报道的八羰基金属配合物 M(CO) 8 (M = Ca, Sr, Ba) 具有有趣的键合结构。在这些化合物中,键级为7,同时容纳8个孤对配体形成八配位络合物或离子。在这里,通过类比地比较 [Ba(CO) 8 ] 2−和 [BaBe 8 ] 2−的金属团簇,我们证明 Jellium 模型不仅可以应用于金属团簇,而且还是理解电子团簇的有用工具[M(CO) 8 ] q的结构(M,q = Ca,2−;Sc,1−;Ti,0;V,1+;Cr,2+;Ba,2−)。通过应用 Jellium 模型,我们发现配置为 |1S 2 |1P的 20-e 模型6 |1D 10 |1F 2 | 是对 M(CO) 8种的价键结构的适当描述,其中每个配位键包含 7/8 的键合轨道和 1/8 的非键合轨道。


























 京公网安备 11010802027423号
京公网安备 11010802027423号