Modern Pathology ( IF 7.5 ) Pub Date : 2020-03-26 , DOI: 10.1038/s41379-020-0529-9 Gyuheon Choi 1 , Young-Gon Kim 2 , Haeyon Cho 1 , Namkug Kim 3 , Hyunna Lee 3 , Kyung Chul Moon 4 , Heounjeong Go 1
|
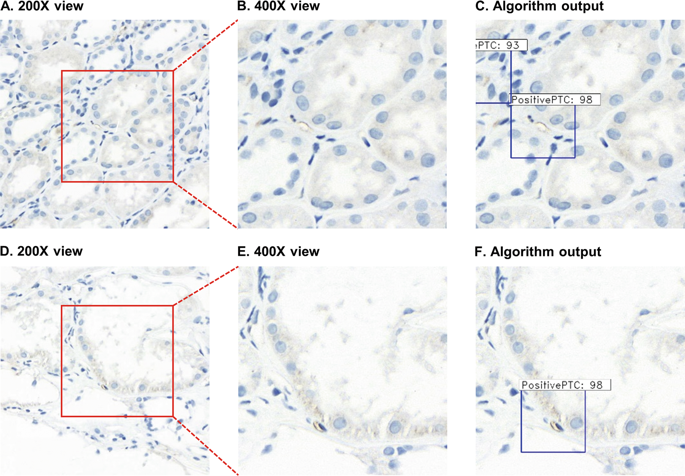
A deep learning-based image analysis could improve diagnostic accuracy and efficiency in pathology work. Recently, we proposed a deep learning-based detection algorithm for C4d immunostaining in renal allografts. The objective of this study is to assess the diagnostic performance of the algorithm by comparing pathologists’ diagnoses and analyzing the associations of the algorithm with clinical data. C4d immunostaining slides of renal allografts were obtained from two different institutions (100 slides from the Asan Medical Center and 86 slides from the Seoul National University Hospital) and scanned using two different slide scanners. Three pathologists and the algorithm independently evaluated each slide according to the Banff 2017 criteria. Subsequently, they jointly reviewed the results for consensus scoring. The result of the algorithm was compared with that of each pathologist and the consensus diagnosis. Clinicopathological associations of the results of the algorithm with allograft survival, histologic evidence of microvascular inflammation, and serologic results for donor-specific antibodies were also analyzed. As a result, the reproducibility between the pathologists was fair to moderate (kappa 0.36–0.54), which is comparable to that between the algorithm and each pathologist (kappa 0.34–0.51). The C4d scores predicted by the algorithm achieved substantial concordance with the consensus diagnosis (kappa = 0.61), and they were significantly associated with remarkable microvascular inflammation (P = 0.001), higher detection rate of donor-specific antibody (P = 0.003), and shorter graft survival (P < 0.001). In conclusion, the deep learning-based C4d detection algorithm showed a diagnostic performance similar to that of the pathologists.
中文翻译:

C4d 免疫染色的自动检测算法在肾同种异体移植活检中显示出与病理学家相当的诊断性能。
基于深度学习的图像分析可以提高病理工作的诊断准确性和效率。最近,我们提出了一种基于深度学习的肾同种异体移植物 C4d 免疫染色检测算法。本研究的目的是通过比较病理学家的诊断并分析算法与临床数据的关联来评估算法的诊断性能。肾同种异体移植物的 C4d 免疫染色载玻片是从两个不同的机构获得的(100 张载玻片来自牙山医疗中心,86 张载玻片来自首尔国立大学医院)并使用两种不同的载玻片扫描仪进行扫描。三位病理学家和算法根据 Banff 2017 标准独立评估每张幻灯片。随后,他们共同审查了共识评分的结果。将算法的结果与每个病理学家的结果和共识诊断进行比较。还分析了算法结果与同种异体移植物存活率、微血管炎症的组织学证据以及供体特异性抗体的血清学结果之间的临床病理学关联。因此,病理学家之间的可重复性一般到中等 (kappa 0.36–0.54),这与算法和每位病理学家之间的可重复性 (kappa 0.34–0.51) 相当。该算法预测的 C4d 分数与共识诊断(kappa = 0.61)基本一致,并且它们与显着的微血管炎症显着相关(还分析了微血管炎症的组织学证据和供体特异性抗体的血清学结果。因此,病理学家之间的可重复性一般到中等 (kappa 0.36–0.54),这与算法和每位病理学家之间的可重复性 (kappa 0.34–0.51) 相当。该算法预测的 C4d 分数与共识诊断(kappa = 0.61)基本一致,并且它们与显着的微血管炎症显着相关(还分析了微血管炎症的组织学证据和供体特异性抗体的血清学结果。因此,病理学家之间的可重复性一般到中等 (kappa 0.36–0.54),这与算法和每位病理学家之间的可重复性 (kappa 0.34–0.51) 相当。该算法预测的 C4d 分数与共识诊断(kappa = 0.61)基本一致,并且它们与显着的微血管炎症显着相关(P = 0.001),供体特异性抗体检出率更高(P = 0.003),移植物存活时间更短(P < 0.001)。总之,基于深度学习的 C4d 检测算法显示出与病理学家相似的诊断性能。



























 京公网安备 11010802027423号
京公网安备 11010802027423号