当前位置:
X-MOL 学术
›
Beilstein. J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Controlling alkyne reactivity by means of a copper-catalyzed radical reaction system for the synthesis of functionalized quaternary carbons
Beilstein Journal of Organic Chemistry ( IF 2.7 ) Pub Date : 2020-03-26 , DOI: 10.3762/bjoc.16.45 Goki Hirata , Yu Yamane , Naoya Tsubaki , Reina Hara , Takashi Nishikata
Beilstein Journal of Organic Chemistry ( IF 2.7 ) Pub Date : 2020-03-26 , DOI: 10.3762/bjoc.16.45 Goki Hirata , Yu Yamane , Naoya Tsubaki , Reina Hara , Takashi Nishikata
|
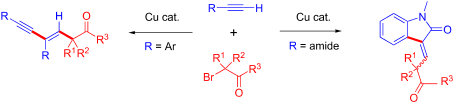
A terminal alkyne is one of the most useful reactants for the synthesis of alkyne and alkene derivatives. Because an alkyne undergoes addition reaction at a C–C triple bond or cross-coupling at a terminal C–H bond. Combining those reaction patterns could realize a new reaction methodology to synthesize complex molecules including C–C multiple bonds. In this report, we found that the reaction of 3 equivalents of terminal alkyne 1 (aryl substituted alkyne) and an α-bromocarbonyl compound 2 (tertiary alkyl radical precursor) undergoes tandem alkyl radical addition/Sonogashira coupling to produce 1,3-enyne compound 3 possessing a quaternary carbon in the presence of a copper catalyst. Moreover, the reaction of α-bromocarbonyl compound 2 and an alkyne 4 possessing a carboxamide moiety undergoes tandem alkyl radical addition/C–H coupling to produce indolinone derivative 5.
中文翻译:

通过铜催化的自由基反应系统控制炔烃反应性,以合成功能化的季碳
末端炔烃是用于合成炔烃和烯烃衍生物的最有用的反应物之一。因为炔烃在C–C三键处发生加成反应,或在C–H末端发生交叉偶联。结合这些反应模式可以实现一种新的反应方法,以合成包括CC多键的复杂分子。在此报告中,我们发现3当量的末端炔烃1(芳基取代的炔烃)与α-溴羰基化合物2(叔烷基自由基前体)的反应经过串联烷基自由基加成/ Sonogashira偶联生成1,3-烯炔化合物在铜催化剂存在下具有季碳的3。此外,α-溴羰基化合物2的反应和具有羧酰胺部分的炔4进行串联烷基自由基加成反应/ CH偶联生成茚满酮衍生物5。
更新日期:2020-03-27
中文翻译:

通过铜催化的自由基反应系统控制炔烃反应性,以合成功能化的季碳
末端炔烃是用于合成炔烃和烯烃衍生物的最有用的反应物之一。因为炔烃在C–C三键处发生加成反应,或在C–H末端发生交叉偶联。结合这些反应模式可以实现一种新的反应方法,以合成包括CC多键的复杂分子。在此报告中,我们发现3当量的末端炔烃1(芳基取代的炔烃)与α-溴羰基化合物2(叔烷基自由基前体)的反应经过串联烷基自由基加成/ Sonogashira偶联生成1,3-烯炔化合物在铜催化剂存在下具有季碳的3。此外,α-溴羰基化合物2的反应和具有羧酰胺部分的炔4进行串联烷基自由基加成反应/ CH偶联生成茚满酮衍生物5。


























 京公网安备 11010802027423号
京公网安备 11010802027423号