Electrochimica Acta ( IF 6.6 ) Pub Date : 2020-03-26 , DOI: 10.1016/j.electacta.2020.136113 Jonathan Moldenhauer , Catherine Sella , Becca Moffett , Joel Baker , Laurent Thouin , Christian Amatore , Stefan M. Kilyanek , David W. Paul
|
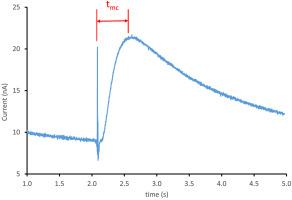
Diffusion coefficients are an important physical property to both physical and analytical electrochemistry and the role they play in sensors, batteries, and catalysis. In electrochemical time of flight (ETOF), the time needed for an electrochemically generated species to travel to an adjacent electrode is measured. Previously relegated to verifying diffusion modeling and theoretical work, ETOF is a powerful and elegant technique whose broad applicability has been overlooked. In the determination of diffusion coefficients, ETOF does not require setting delicate hydrodynamic conditions required by rotating ring disk (RDE) methods. In addition, no foreknowledge about the complete electrochemical mechanistic system, nor about its electron stoichiometry is required. ETOF is ideal for determining the diffusion coefficients for non-elucidated systems, such as for short-lived intermediates assuming they survive ETOF travel time. Here we report the construction of a universal empirical calibration curve for the determination of diffusion coefficients using experimental parameters optimized by computer modeling. Using a platinum micro electrode array, diffusion coefficients were determined for a variety of species including ferrocenium, ruthenium (III) bisbipyridine dichloride, and vanadium (III) acetylacetonate, some of which being previously unreported. The elegance of the method is that the calibration curve constructed with a few well-established species in a given electrolyte can be used for any species in any electrolyte.
中文翻译:

优化电化学飞行时间测量,以精确测定各种介质中的扩散系数
扩散系数是物理和分析电化学的重要物理性质,以及它们在传感器,电池和催化中的作用。在电化学飞行时间(ETOF)中,测量了电化学产生的物质移动到相邻电极所需的时间。ETOF以前只属于验证扩散模型和理论工作的领域,它是一种功能强大且优雅的技术,其广泛的适用性已被忽略。在确定扩散系数时,ETOF不需要设置旋转环盘(RDE)方法所需的精密流体动力学条件。另外,不需要关于完整的电化学机制系统的知识,也不需要关于其电子化学计量的知识。ETOF是确定未阐明系统的扩散系数的理想选择,例如对于短寿命的中间体,假设它们在ETOF的运行时间内仍能存活。在这里,我们报告了通过计算机建模优化的实验参数确定扩散系数的通用经验校准曲线的构建。使用铂微电极阵列,确定了各种元素的扩散系数,包括二茂铁,钌(III)双联吡啶二氯化物和乙酰丙酮钒(III),其中一些以前没有报道。该方法的精妙之处在于,在给定的电解质中用几个公认的物质构建的校准曲线可用于任何电解质中的任何物质。在这里,我们报告了使用计算机建模优化的实验参数确定扩散系数的通用经验校准曲线的构建。使用铂微电极阵列,确定了各种元素的扩散系数,包括二茂铁,钌(III)双联吡啶二氯化物和乙酰丙酮钒(III),其中一些以前没有报道。该方法的精妙之处在于,在给定的电解质中用几个公认的物质构建的校准曲线可用于任何电解质中的任何物质。在这里,我们报告了使用计算机建模优化的实验参数确定扩散系数的通用经验校准曲线的构建。使用铂微电极阵列,确定了各种元素的扩散系数,包括二茂铁,钌(III)双联吡啶二氯化物和乙酰丙酮钒(III),其中一些以前没有报道。该方法的精妙之处在于,在给定的电解质中用几个公认的物质构建的校准曲线可用于任何电解质中的任何物质。钌(III)双联吡啶二氯化物和钒(III)乙酰丙酮钒,其中一些以前未报道。该方法的精妙之处在于,在给定的电解质中用几个公认的物质构建的校准曲线可用于任何电解质中的任何物质。钌(III)双联吡啶二氯化物和钒(III)乙酰丙酮钒,其中一些以前未报道。该方法的精妙之处在于,在给定的电解质中用几个公认的物质构建的校准曲线可用于任何电解质中的任何物质。



























 京公网安备 11010802027423号
京公网安备 11010802027423号