Electrochimica Acta ( IF 6.6 ) Pub Date : 2020-03-26 , DOI: 10.1016/j.electacta.2020.136122 Shishir Kumar Singh , Dimple Dutta , Rajendra Kumar Singh
|
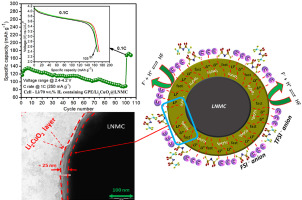
Recently, ionic liquid-based gel polymer electrolytes (IL-GPEs) attracted increasing attention because of its use in developing safe and flexible rechargeable lithium-based batteries. The IL-GPE composed of microporous polymer PVdF-HFP incorporating different weight percentages of ionic liquid PYR13FSI with 20 wt% lithium salt LiTFSI is prepared. These prepared films are investigated in detail by thermogravimetric analysis, impedance spectroscopy, cyclic voltammetry and linear sweep voltammetry measurements for battery application. The 70 wt% IL containing GPE shows excellent thermal stability up to ∼240 °C and high lithium ion conductivity (1.6 × 10−3 S cm−1 at 30 °C) with wide electrochemical stability window (∼4.3 V vs. Li/Li+ at 30 °C). Furthermore, the Li+-ion conductive Li2CuO2 is coated on LiNi0.33Mn0.33Co0.33O2 (Li2CuO2@LNMC) cathode particle by using a wet chemical method. The structural and electrochemical properties of pristine and Li2CuO2@LNMC cathode are investigated using XRD, SEM, TEM and electrochemical analysis. The XRD pattern shows that no impurity phase is present in the Li2CuO2@LNMC cathode. Thin Li2CuO2 coating layer (20–25 nm) on the surface of LNMC particle is confirmed by TEM image. The optimized electrolyte is used to fabricate Li-cells (Li/LNMC and Li/Li2CuO2@LNMC). The charge-discharge results show that the initial specific discharge capacity of Li2CuO2@LNMC is ∼196 mAh g−1 at 0.1C (25 mA g−1), whereas, the pristine LNMC has ∼182 mAh g−1 under same condition. The Li2CuO2 coating layer improves electrochemical performance and cyclic stability of pristine cathode up to 100th cycles at 1 C-rate. The capacity retention is ∼69% for Li2CuO2@LNMC over 100th charge-discharge cycles at 1C, whereas, the capacity retention is only ∼30% for pristine LNMC. After cycling, the EIS results also indicate that the impedance of Li/LNMC cell reduces after Li2CuO2 modification.
中文翻译:

锂聚合物电池用柔性离子液体基凝胶聚合物电解质增强Li 2 CuO 2包覆的LiNi 0.33 Mn 0.33 Co 0.33 O 2阴极的结构和循环稳定性
近年来,由于离子液体基凝胶聚合物电解质(IL-GPE)用于开发安全,灵活的可充电锂基电池,因此受到越来越多的关注。制备由微孔聚合物PVdF-HFP组成的IL-GPE,该微孔聚合物结合了不同重量百分比的离子液体PYR 13 FSI和20 wt%的锂盐LiTFSI。通过热重分析,阻抗谱,循环伏安法和线性扫描伏安法测量这些制备的薄膜,以用于电池应用。含70 wt%IL的GPE在高达约240°C的温度下具有出色的热稳定性,并且锂离子传导率高(30°C时为1.6×10 -3 S cm -1),电化学稳定性窗口宽(约4.3 V vs. Li /李+在30°C下)。此外,通过使用湿化学方法,将Li +离子导电性Li 2 CuO 2涂覆在LiNi 0.33 Mn 0.33 Co 0.33 O 2(Li 2 CuO 2 @LNMC)阴极颗粒上。利用XRD,SEM,TEM和电化学分析研究了原始的Li 2 CuO 2 @LNMC阴极的结构和电化学性能。XRD图谱表明在Li 2 CuO 2 @LNMC阴极中不存在杂质相。稀薄的Li 2 CuO 2通过TEM图像确认LNMC颗粒表面的涂层(20–25 nm)。优化的电解质用于制造锂电池(Li / LNMC和Li / Li 2 CuO 2 @LNMC)。充放电结果表明,Li的初始放电比容量2的CuO 2 @LNMC是~196毫安克-1在0.1C(25毫安克-1),而,原始LNMC具有~182毫安克-1下同样的条件。Li 2 CuO 2涂层在1 C速率下可提高原始阴极的电化学性能和循环稳定性,直至第100个循环。Li 2 CuO 2的容量保持率为〜69%@LNMC在1C的第100次充放电循环中,而原始LNMC的容量保持率仅为〜30%。循环后,EIS结果还表明,Li 2 CuO 2改性后,Li / LNMC电池的阻抗降低。



























 京公网安备 11010802027423号
京公网安备 11010802027423号