当前位置:
X-MOL 学术
›
ACS Med. Chem. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Design of First-in-Class Dual EZH2/HDAC Inhibitor: Biochemical Activity and Biological Evaluation in Cancer Cells.
ACS Medicinal Chemistry Letters ( IF 4.2 ) Pub Date : 2020-03-19 , DOI: 10.1021/acsmedchemlett.0c00014 Annalisa Romanelli 1 , Giulia Stazi 1 , Rossella Fioravanti 1 , Clemens Zwergel 1, 2 , Elisabetta Di Bello 1 , Silvia Pomella 3 , Clara Perrone 3 , Cecilia Battistelli 4 , Raffaele Strippoli 4, 5 , Marco Tripodi 4, 5 , Donatella Del Bufalo 6 , Rossella Rota 3 , Daniela Trisciuoglio 6, 7 , Antonello Mai 1 , Sergio Valente 1
ACS Medicinal Chemistry Letters ( IF 4.2 ) Pub Date : 2020-03-19 , DOI: 10.1021/acsmedchemlett.0c00014 Annalisa Romanelli 1 , Giulia Stazi 1 , Rossella Fioravanti 1 , Clemens Zwergel 1, 2 , Elisabetta Di Bello 1 , Silvia Pomella 3 , Clara Perrone 3 , Cecilia Battistelli 4 , Raffaele Strippoli 4, 5 , Marco Tripodi 4, 5 , Donatella Del Bufalo 6 , Rossella Rota 3 , Daniela Trisciuoglio 6, 7 , Antonello Mai 1 , Sergio Valente 1
Affiliation
|
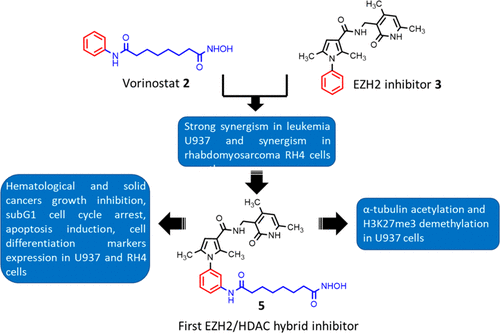
Since the histone modifying enzymes EZH2 and HDACs control a number of epigenetic-dependent carcinogenic pathways, we designed the first-in-class dual EZH2/HDAC inhibitor 5 displaying (sub)micromolar inhibition against both targets. When tested in several cancer cell lines, the hybrid 5 impaired cell viability at low micromolar level and in leukemia U937 and rhabdomyosarcoma RH4 cells provided G1 arrest, apoptotic induction, and increased differentiation, associated with an increase of acetyl-H3 and acetyl-α-tubulin and a decrease of H3K27me3 levels. In glioblastoma U87 cells, 5 hampered epithelial to mesenchymal transition by increasing the E-cadherin expression, thus proposing itself as a useful candidate for anticancer therapy.
中文翻译:

一流的双重EZH2 / HDAC抑制剂设计:癌细胞的生化活性和生物学评估。
由于组蛋白修饰酶EZH2和HDAC控制许多表观遗传依赖性致癌途径,因此我们设计了同类中第一个双重EZH2 / HDAC抑制剂5,对两个靶标均表现出(亚)微摩尔抑制作用。当在几种癌细胞系中进行测试时,杂合体5在低微摩尔水平以及白血病U937和横纹肌肉瘤RH4细胞中损害了细胞活力,提供了G1阻滞,凋亡诱导和分化增强,并伴有乙酰H3和乙酰α-的增加。微管蛋白和H3K27me3水平降低。在胶质母细胞瘤U87细胞中,有5种通过增加E-钙黏着蛋白的表达而阻碍了上皮向间充质的转化,因此,其自身有望成为抗癌治疗的有用候选药物。
更新日期:2020-03-19
中文翻译:

一流的双重EZH2 / HDAC抑制剂设计:癌细胞的生化活性和生物学评估。
由于组蛋白修饰酶EZH2和HDAC控制许多表观遗传依赖性致癌途径,因此我们设计了同类中第一个双重EZH2 / HDAC抑制剂5,对两个靶标均表现出(亚)微摩尔抑制作用。当在几种癌细胞系中进行测试时,杂合体5在低微摩尔水平以及白血病U937和横纹肌肉瘤RH4细胞中损害了细胞活力,提供了G1阻滞,凋亡诱导和分化增强,并伴有乙酰H3和乙酰α-的增加。微管蛋白和H3K27me3水平降低。在胶质母细胞瘤U87细胞中,有5种通过增加E-钙黏着蛋白的表达而阻碍了上皮向间充质的转化,因此,其自身有望成为抗癌治疗的有用候选药物。


























 京公网安备 11010802027423号
京公网安备 11010802027423号