当前位置:
X-MOL 学术
›
J. Med. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Design, Synthesis, and Biological Evaluation of Quinazolin-4-one-Based Hydroxamic Acids as Dual PI3K/HDAC Inhibitors.
Journal of Medicinal Chemistry ( IF 7.3 ) Pub Date : 2020-04-08 , DOI: 10.1021/acs.jmedchem.0c00193 Ashish Thakur 1 , Gregory J Tawa 1 , Mark J Henderson 1 , Carina Danchik 1 , Suiyang Liu 2 , Pranav Shah 1 , Amy Q Wang 1 , Garrett Dunn 1 , Md Kabir 1 , Elias C Padilha 1 , Xin Xu 1 , Anton Simeonov 1 , Surender Kharbanda 2 , Richard Stone 2 , Gurmit Grewal 1
Journal of Medicinal Chemistry ( IF 7.3 ) Pub Date : 2020-04-08 , DOI: 10.1021/acs.jmedchem.0c00193 Ashish Thakur 1 , Gregory J Tawa 1 , Mark J Henderson 1 , Carina Danchik 1 , Suiyang Liu 2 , Pranav Shah 1 , Amy Q Wang 1 , Garrett Dunn 1 , Md Kabir 1 , Elias C Padilha 1 , Xin Xu 1 , Anton Simeonov 1 , Surender Kharbanda 2 , Richard Stone 2 , Gurmit Grewal 1
Affiliation
|
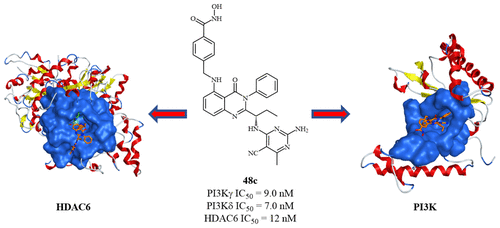
A series of quinazolin-4-one based hydroxamic acids was rationally designed and synthesized as novel dual PI3K/HDAC inhibitors by incorporating an HDAC pharmacophore into a PI3K inhibitor (Idelalisib) via an optimized linker. Several of these dual inhibitors were highly potent (IC50 < 10 nM) and selective against PI3Kγ, δ and HDAC6 enzymes and exhibited good antiproliferative activity against multiple cancer cell lines. The lead compound 48c, induced necrosis in several mutant and FLT3-resistant AML cell lines and primary blasts from AML patients, while showing no cytotoxicity against normal PBMCs, NIH3T3, and HEK293 cells. Target engagement of PI3Kδ and HDAC6 by 48c was demonstrated in MV411 cells using the cellular thermal shift assay (CETSA). Compound 48c showed good pharmacokinetics properties in mice via intraperitoneal (ip) administration and provides a means to examine the biological effects of inhibiting these two important enzymes with a single molecule, either in vitro or in vivo.
中文翻译:

喹唑啉-4-酮基异羟肟酸作为PI3K / HDAC双重抑制剂的设计,合成和生物学评估。
通过经由优化的接头将HDAC药效团掺入PI3K抑制剂(Idelalisib)中,合理设计和合成了一系列基于喹唑啉-4-酮的异羟肟酸,作为新型的双重PI3K / HDAC抑制剂。这些双重抑制剂中的几种非常有效(IC50 <10 nM),对PI3Kγ,δ和HDAC6酶具有选择性,并且对多种癌细胞具有良好的抗增殖活性。铅化合物48c在AML患者的几种突变型和FLT3抗性AML细胞系和原代细胞中诱导坏死,而对正常PBMC,NIH3T3和HEK293细胞无细胞毒性。使用细胞热位移分析(CETSA)在MV411细胞中证明了48c的PI3Kδ和HDAC6的靶标结合。
更新日期:2020-04-24
中文翻译:

喹唑啉-4-酮基异羟肟酸作为PI3K / HDAC双重抑制剂的设计,合成和生物学评估。
通过经由优化的接头将HDAC药效团掺入PI3K抑制剂(Idelalisib)中,合理设计和合成了一系列基于喹唑啉-4-酮的异羟肟酸,作为新型的双重PI3K / HDAC抑制剂。这些双重抑制剂中的几种非常有效(IC50 <10 nM),对PI3Kγ,δ和HDAC6酶具有选择性,并且对多种癌细胞具有良好的抗增殖活性。铅化合物48c在AML患者的几种突变型和FLT3抗性AML细胞系和原代细胞中诱导坏死,而对正常PBMC,NIH3T3和HEK293细胞无细胞毒性。使用细胞热位移分析(CETSA)在MV411细胞中证明了48c的PI3Kδ和HDAC6的靶标结合。


























 京公网安备 11010802027423号
京公网安备 11010802027423号