当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Total Synthesis of Nominal ent-Chlorabietol B
The Journal of Organic Chemistry ( IF 3.6 ) Pub Date : 2020-03-26 , DOI: 10.1021/acs.joc.0c00233 Yulong Li 1 , Zhezhe Xu 1 , Zhipeng Xie 1 , Xingchao Guan 1 , Zhixiang Xie 1
The Journal of Organic Chemistry ( IF 3.6 ) Pub Date : 2020-03-26 , DOI: 10.1021/acs.joc.0c00233 Yulong Li 1 , Zhezhe Xu 1 , Zhipeng Xie 1 , Xingchao Guan 1 , Zhixiang Xie 1
Affiliation
|
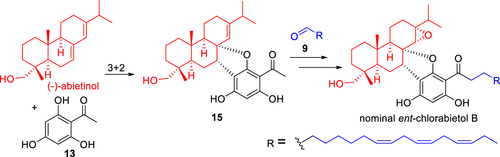
The nominal enantiomer of chlorabietol B was regio- and stereoselectively synthesized from (−)-abietic acid in 13 steps. Key features of the synthesis involved an oxidative [3+2] cycloaddition to install the dihydrobenzofuran moiety and an Aldol reaction, followed by elimination and reduction steps to introduce the long chain with three cis double bonds. However, obvious differences in the NMR spectra of the synthetic and natural samples suggested that the proposed structure of chlorabietol B should be revised carefully.
中文翻译:

名义对氯胆甾醇B的全合成
从(-)-松香酸经13个步骤区域和立体选择性合成了氯丁醇B的标称对映体。合成的关键特征包括氧化[3 + 2]环加成反应以安装二氢苯并呋喃部分和Aldol反应,然后进行消除和还原步骤以引入具有三个顺式双键的长链。但是,合成样品和天然样品在NMR谱图中的明显差异表明,应谨慎修改拟议的氯丁醇B的结构。
更新日期:2020-04-24
中文翻译:

名义对氯胆甾醇B的全合成
从(-)-松香酸经13个步骤区域和立体选择性合成了氯丁醇B的标称对映体。合成的关键特征包括氧化[3 + 2]环加成反应以安装二氢苯并呋喃部分和Aldol反应,然后进行消除和还原步骤以引入具有三个顺式双键的长链。但是,合成样品和天然样品在NMR谱图中的明显差异表明,应谨慎修改拟议的氯丁醇B的结构。


























 京公网安备 11010802027423号
京公网安备 11010802027423号