当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Expressed Protein Ligation Without Intein
Journal of the American Chemical Society ( IF 15.0 ) Pub Date : 2020-03-26 , DOI: 10.1021/jacs.0c00252 Yuchen Qiao 1 , Ge Yu 1 , Kaci C Kratch 1 , Xiaoyan Aria Wang 1 , Wesley Wei Wang 1 , Sunshine Z Leeuwon 1 , Shiqing Xu 1 , Jared S Morse 1 , Wenshe Ray Liu 1, 2, 3
Journal of the American Chemical Society ( IF 15.0 ) Pub Date : 2020-03-26 , DOI: 10.1021/jacs.0c00252 Yuchen Qiao 1 , Ge Yu 1 , Kaci C Kratch 1 , Xiaoyan Aria Wang 1 , Wesley Wei Wang 1 , Sunshine Z Leeuwon 1 , Shiqing Xu 1 , Jared S Morse 1 , Wenshe Ray Liu 1, 2, 3
Affiliation
|
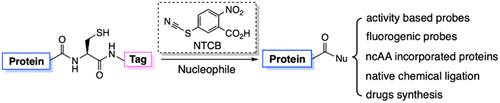
Proteins with a functionalized C-terminus such as a C-terminal thioester are key to the synthesis of larger proteins via expressed protein ligation. They are usually made by re-combinant fusion to intein. Although powerful, the intein fusion approach suffers from premature hydrolysis and low compatibility with denatured conditions. To totally bypass the involvement of an enzyme for expressed protein ligation, here we showed that a cysteine in a recombinant protein was chemically activated by a small molecule cyanylating reagent at its N-side amide for undergoing nucleophilic acyl substitu-tion with amines including a number of L- and D-amino acids and hydrazine. The afforded protein hydrazides could be used further for expressed protein ligation. We demonstrated the versatility of this activated cysteine-directed protein ligation (ACPL) approach with the successful synthesis of ubiquitin conjugates, ubiquitin-like protein conjugates, histone H2A with a C-terminal posttranslational modification, RNAse H that actively hydrolyzed RNA, and exenatide that is a com-mercial therapeutic peptide. The technique, which is exceed-ingly simple but highly useful, expands to a great extent the synthetic capacity of protein chemistry and will therefore make a large avenue of new research possible.
中文翻译:

不含内含肽的表达蛋白连接
具有功能化 C 末端(例如 C 末端硫酯)的蛋白质是通过表达的蛋白质连接合成较大蛋白质的关键。它们通常是通过与内含肽重组融合而制成的。虽然功能强大,但内含肽融合方法存在过早水解和与变性条件的相容性低的问题。为了完全绕过用于表达蛋白连接的酶的参与,我们在这里展示了重组蛋白中的半胱氨酸在其 N 侧酰胺处被小分子氰化试剂化学活化,以进行亲核酰基取代,包括一些胺L-和D-氨基酸和肼。得到的蛋白酰肼可进一步用于表达蛋白的连接。我们通过成功合成泛素偶联物、泛素样蛋白质偶联物、具有 C 端翻译后修饰的组蛋白 H2A、主动水解 RNA 的 RNAse H 和艾塞那肽,证明了这种活化的半胱氨酸定向蛋白连接 (ACPL) 方法的多功能性是一种商业治疗肽。该技术非常简单但非常有用,在很大程度上扩展了蛋白质化学的合成能力,因此将使新的研究成为可能。
更新日期:2020-03-26
中文翻译:

不含内含肽的表达蛋白连接
具有功能化 C 末端(例如 C 末端硫酯)的蛋白质是通过表达的蛋白质连接合成较大蛋白质的关键。它们通常是通过与内含肽重组融合而制成的。虽然功能强大,但内含肽融合方法存在过早水解和与变性条件的相容性低的问题。为了完全绕过用于表达蛋白连接的酶的参与,我们在这里展示了重组蛋白中的半胱氨酸在其 N 侧酰胺处被小分子氰化试剂化学活化,以进行亲核酰基取代,包括一些胺L-和D-氨基酸和肼。得到的蛋白酰肼可进一步用于表达蛋白的连接。我们通过成功合成泛素偶联物、泛素样蛋白质偶联物、具有 C 端翻译后修饰的组蛋白 H2A、主动水解 RNA 的 RNAse H 和艾塞那肽,证明了这种活化的半胱氨酸定向蛋白连接 (ACPL) 方法的多功能性是一种商业治疗肽。该技术非常简单但非常有用,在很大程度上扩展了蛋白质化学的合成能力,因此将使新的研究成为可能。



























 京公网安备 11010802027423号
京公网安备 11010802027423号