Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
The failure of microglia to digest developmental apoptotic cells contributes to the pathology of RNASET2-deficient leukoencephalopathy.
Glia ( IF 6.2 ) Pub Date : 2020-03-25 , DOI: 10.1002/glia.23829 Noémie Hamilton 1 , Holly A Rutherford 1 , Jessica J Petts 1 , Hannah M Isles 1 , Thomas Weber 2 , Marco Henneke 2 , Jutta Gärtner 2 , Mark J Dunning 3 , Stephen A Renshaw 1
Glia ( IF 6.2 ) Pub Date : 2020-03-25 , DOI: 10.1002/glia.23829 Noémie Hamilton 1 , Holly A Rutherford 1 , Jessica J Petts 1 , Hannah M Isles 1 , Thomas Weber 2 , Marco Henneke 2 , Jutta Gärtner 2 , Mark J Dunning 3 , Stephen A Renshaw 1
Affiliation
|
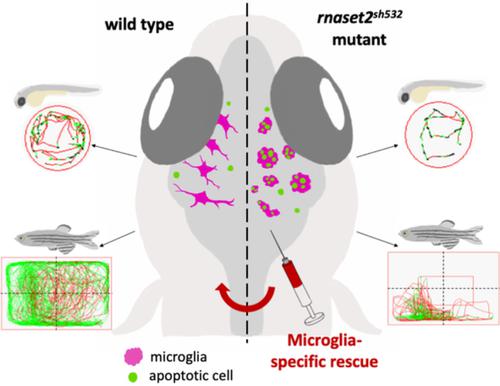
The contribution of microglia in neurological disorders is emerging as a leading disease driver rather than a consequence of pathology. RNAseT2-deficient leukoencephalopathy is a severe childhood white matter disorder affecting patients in their first year of life and mimicking a cytomegalovirus brain infection. The early onset and resemblance of the symptoms to a viral infection suggest an inflammatory and embryonic origin of the pathology. There are no treatments available for this disease as our understanding of the cellular drivers of the pathology are still unknown. In this study, using a zebrafish mutant for the orthologous rnaset2 gene, we have identified an inflammatory signature in early development and an antiviral immune response in mature adult brains. Using the optical transparency and the ex utero development of the zebrafish larvae we studied immune cell behavior during brain development and identified abnormal microglia as an early marker of pathology. Live imaging and electron microscopy identified that mutant microglia displayed an engorged morphology and were filled with undigested apoptotic cells and undigested substrate. Using microglia-specific depletion and rescue experiments, we identified microglia as drivers of this embryonic phenotype and potential key cellular player in the pathology of RNAseT2-deficient leukoencephalopathy. Our zebrafish model also presented with reduced survival and locomotor defects, therefore recapitulating many aspects of the human disease. Our study therefore placed our rnaset2 mutant at the forefront of leukodystrophy preclinical models and highlighted tissue-specific approaches as future therapeutic avenues.
中文翻译:

小胶质细胞无法消化发育中的凋亡细胞导致 RNASET2 缺陷型白质脑病的病理学。
小胶质细胞在神经系统疾病中的贡献正在成为主要的疾病驱动因素,而不是病理学的结果。RNAseT2-deficient leukoencephalopathy 是一种严重的儿童白质疾病,影响患者在其生命的第一年,并模仿巨细胞病毒脑部感染。症状的早期发作和与病毒感染的相似性表明病理学的炎症和胚胎起源。由于我们对病理学的细胞驱动因素的理解仍然未知,因此没有可用于这种疾病的治疗方法。在这项研究中,我们使用直系同源 rnaset2 基因的斑马鱼突变体,确定了早期发育中的炎症特征和成熟成人大脑中的抗病毒免疫反应。利用斑马鱼幼虫的光学透明度和体外发育,我们研究了大脑发育过程中的免疫细胞行为,并将异常小胶质细胞确定为病理的早期标志物。实时成像和电子显微镜发现突变的小胶质细胞表现出充血的形态,并充满了未消化的凋亡细胞和未消化的底物。使用小胶质细胞特异性耗竭和拯救实验,我们确定小胶质细胞是这种胚胎表型的驱动因素,也是 RNAseT2 缺陷型白质脑病病理学中潜在的关键细胞参与者。我们的斑马鱼模型还呈现出存活率降低和运动缺陷,因此概括了人类疾病的许多方面。
更新日期:2020-03-25
中文翻译:

小胶质细胞无法消化发育中的凋亡细胞导致 RNASET2 缺陷型白质脑病的病理学。
小胶质细胞在神经系统疾病中的贡献正在成为主要的疾病驱动因素,而不是病理学的结果。RNAseT2-deficient leukoencephalopathy 是一种严重的儿童白质疾病,影响患者在其生命的第一年,并模仿巨细胞病毒脑部感染。症状的早期发作和与病毒感染的相似性表明病理学的炎症和胚胎起源。由于我们对病理学的细胞驱动因素的理解仍然未知,因此没有可用于这种疾病的治疗方法。在这项研究中,我们使用直系同源 rnaset2 基因的斑马鱼突变体,确定了早期发育中的炎症特征和成熟成人大脑中的抗病毒免疫反应。利用斑马鱼幼虫的光学透明度和体外发育,我们研究了大脑发育过程中的免疫细胞行为,并将异常小胶质细胞确定为病理的早期标志物。实时成像和电子显微镜发现突变的小胶质细胞表现出充血的形态,并充满了未消化的凋亡细胞和未消化的底物。使用小胶质细胞特异性耗竭和拯救实验,我们确定小胶质细胞是这种胚胎表型的驱动因素,也是 RNAseT2 缺陷型白质脑病病理学中潜在的关键细胞参与者。我们的斑马鱼模型还呈现出存活率降低和运动缺陷,因此概括了人类疾病的许多方面。



























 京公网安备 11010802027423号
京公网安备 11010802027423号