当前位置:
X-MOL 学术
›
Adv. Synth. Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Anion‐Dependent Imidazolium‐Based Catalysts for Allylation of Aniline with Tunable Regioselectivity
Advanced Synthesis & Catalysis ( IF 5.4 ) Pub Date : 2020-03-24 , DOI: 10.1002/adsc.202000102 María Albert‐Soriano 1 , Isidro M. Pastor 1
Advanced Synthesis & Catalysis ( IF 5.4 ) Pub Date : 2020-03-24 , DOI: 10.1002/adsc.202000102 María Albert‐Soriano 1 , Isidro M. Pastor 1
Affiliation
|
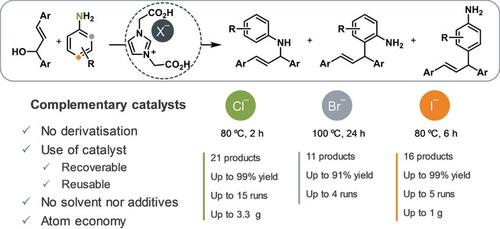
Metal‐free catalysts based on 1,3‐bis(carboxymethyl)imidazolium halides mediate the reaction between allylic alcohols and anilines, providing the corresponding N ‐, 2‐ and 4‐allylaniline isomers selectively. The imidazolium counterion plays a crucial role in the outcome of the reaction. Thus, while the imidazolium chloride selectively provides the N ‐substituted aniline, the bromide and iodide imidazolium salts produce the 2‐ and 4‐allylaniline isomers, respectively, with excellent selectivities. A set of complementary catalysts, which are available by simple modulation, is here presented to conduct a highly regioselective allylation reaction of anilines. Not only the catalysts are synthesized in a straightforward and easily scalable manner, but they can be recycled and used under solvent‐free reaction condition, due to the favorable interactions with the reactants.
中文翻译:

阴离子依赖性咪唑基催化剂用于苯胺的烯丙基化和可调节的区域选择性
基于1,3-双(羧甲基)咪唑鎓卤化物的无金属催化剂介导烯丙醇与苯胺之间的反应,选择性地提供相应的N-,2-和4-烯丙胺异构体。咪唑鎓抗衡离子在反应结果中起关键作用。因此,尽管咪唑氯化物选择性地提供了N溴代苯胺和碘代咪唑鎓盐是经取代的苯胺,分别产生2和4烯丙基苯胺异构体,具有极好的选择性。本文介绍了一组可通过简单调节获得的互补催化剂,以进行苯胺的高度区域选择性烯丙基化反应。不仅催化剂以简单,易于扩展的方式合成,而且由于与反应物之间的良好相互作用,因此可以在无溶剂的反应条件下进行回收利用。
更新日期:2020-03-24
中文翻译:

阴离子依赖性咪唑基催化剂用于苯胺的烯丙基化和可调节的区域选择性
基于1,3-双(羧甲基)咪唑鎓卤化物的无金属催化剂介导烯丙醇与苯胺之间的反应,选择性地提供相应的N-,2-和4-烯丙胺异构体。咪唑鎓抗衡离子在反应结果中起关键作用。因此,尽管咪唑氯化物选择性地提供了N溴代苯胺和碘代咪唑鎓盐是经取代的苯胺,分别产生2和4烯丙基苯胺异构体,具有极好的选择性。本文介绍了一组可通过简单调节获得的互补催化剂,以进行苯胺的高度区域选择性烯丙基化反应。不仅催化剂以简单,易于扩展的方式合成,而且由于与反应物之间的良好相互作用,因此可以在无溶剂的反应条件下进行回收利用。



























 京公网安备 11010802027423号
京公网安备 11010802027423号