当前位置:
X-MOL 学术
›
Chem. Eur. J.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Highly Polarized Coumarin Derivatives Revisited: Solvent-Controlled Competition Between Proton-Coupled Electron Transfer and Twisted Intramolecular Charge Transfer.
Chemistry - A European Journal ( IF 4.3 ) Pub Date : 2020-03-25 , DOI: 10.1002/chem.202001079 Olaf W Morawski 1 , Łukasz Kielesiński 1, 2 , Daniel T Gryko 2 , Andrzej L Sobolewski 1
Chemistry - A European Journal ( IF 4.3 ) Pub Date : 2020-03-25 , DOI: 10.1002/chem.202001079 Olaf W Morawski 1 , Łukasz Kielesiński 1, 2 , Daniel T Gryko 2 , Andrzej L Sobolewski 1
Affiliation
|
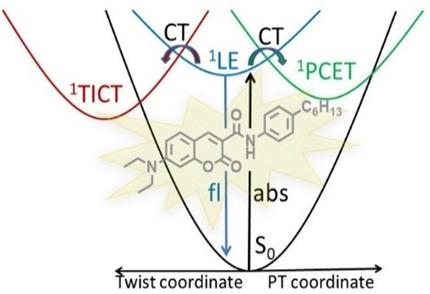
Linking a polarized coumarin unit with an aromatic substituent via an amide bridge results in weak electronic coupling that affects the intramolecular electron‐transfer (ET) process. As a result of this, interesting solvent‐dependent photophysical properties can be observed. In polar solvents, electron transfer in coumarin derivatives of this type induces a mutual twist of the electron‐donating and ‐accepting molecular units (TICT process) that facilitates radiationless decay processes (internal conversion). In the dyad with the strongest intramolecular hydrogen bond, the planar form is stabilized, such that twisting can only occur in highly polar solvents, whereas a fast proton‐coupled electron‐transfer (PCET process) occurs in nonpolar n ‐alkanes. The k PCET rate constant decreases linearly with the energy of the fluorescence maximum in different solvents. This observation can be explained in terms of competition between electron‐ and proton‐transfer from a highly polarized (ca. 15 D) and fluorescent locally excited (1LE) state to a much less polarized (ca. 4 D) charge‐transfer (1CT) state, a unique occurrence. Photophysical measurements performed for a family of related coumarin dyads, together with results of quantum‐chemical computations, give insight into the mechanism of the ET process, which is followed by either a TICT or a PCET process. Our results reveal that dielectric solvation of the excited state slows down the PCET process, even in nonpolar solvents.
中文翻译:

重极化的香豆素衍生物:质子耦合电子转移和扭曲分子内电荷转移之间的溶剂控制竞争。
通过酰胺桥将极化香豆素单元与芳族取代基连接会导致弱电子耦合,从而影响分子内电子转移(ET)过程。结果,可以观察到有趣的溶剂依赖性光物理性质。在极性溶剂中,此类香豆素衍生物中的电子转移会引起给电子和接受电子的分子单元(TICT过程)的相互扭曲,从而促进无辐射衰变过程(内部转化)。在分子内氢键最强的二元化合物中,平面形式是稳定的,因此只能在高极性溶剂中发生扭曲,而在非极性正构烷烃中发生快速的质子耦合电子转移(PCET过程)。该ķ PCET在不同溶剂中,速率常数随荧光最大能量线性降低。可以用电子转移和质子转移从高极化(约15 D)和荧光局部激发(1 LE)状态转变为低极化(约4 D)的电荷转移(1 CT)状态,唯一发生。对一系列相关香豆素二元化合物进行的光物理测量,以及量子化学计算的结果,可以洞悉ET过程的机理,然后是TICT或PCET过程。我们的结果表明,即使在非极性溶剂中,激发态的电介质溶剂化也会减慢PCET过程。
更新日期:2020-03-25
中文翻译:

重极化的香豆素衍生物:质子耦合电子转移和扭曲分子内电荷转移之间的溶剂控制竞争。
通过酰胺桥将极化香豆素单元与芳族取代基连接会导致弱电子耦合,从而影响分子内电子转移(ET)过程。结果,可以观察到有趣的溶剂依赖性光物理性质。在极性溶剂中,此类香豆素衍生物中的电子转移会引起给电子和接受电子的分子单元(TICT过程)的相互扭曲,从而促进无辐射衰变过程(内部转化)。在分子内氢键最强的二元化合物中,平面形式是稳定的,因此只能在高极性溶剂中发生扭曲,而在非极性正构烷烃中发生快速的质子耦合电子转移(PCET过程)。该ķ PCET在不同溶剂中,速率常数随荧光最大能量线性降低。可以用电子转移和质子转移从高极化(约15 D)和荧光局部激发(1 LE)状态转变为低极化(约4 D)的电荷转移(1 CT)状态,唯一发生。对一系列相关香豆素二元化合物进行的光物理测量,以及量子化学计算的结果,可以洞悉ET过程的机理,然后是TICT或PCET过程。我们的结果表明,即使在非极性溶剂中,激发态的电介质溶剂化也会减慢PCET过程。


























 京公网安备 11010802027423号
京公网安备 11010802027423号