当前位置:
X-MOL 学术
›
Org. Biomol. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Stereoselective preparation of P,axial-stereogenic allenyl bisphosphine oxides via chirality-transfer.
Organic & Biomolecular Chemistry ( IF 3.2 ) Pub Date : 2020-04-29 , DOI: 10.1039/d0ob00390e Mao-Ran Qiu 1 , Hong-Xing Zheng 1 , Jing-Jing Ye 1 , Bing-Xia Yan 1 , Chang-Qiu Zhao 1 , Qiang Li 1
Organic & Biomolecular Chemistry ( IF 3.2 ) Pub Date : 2020-04-29 , DOI: 10.1039/d0ob00390e Mao-Ran Qiu 1 , Hong-Xing Zheng 1 , Jing-Jing Ye 1 , Bing-Xia Yan 1 , Chang-Qiu Zhao 1 , Qiang Li 1
Affiliation
|
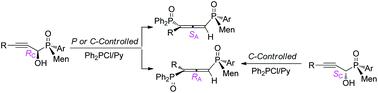
P,C-Stereogenic propargyl alcohols RC-3/SC-3' were prepared by the addition of (L)-menthyl-derived SPOs to propynals, which were converted to P,axial-stereogenic allenyl bisphosphine oxides. The chirality transfer was controlled by α-carbon via syn [2,3]-sigmatropic rearrangement. For SC-3' linking weak WDG on the alkynyl moiety, the chirality on the axis depended on stereogenic phosphorus.
中文翻译:

通过手性转移立体选择性地制备P,轴向立体异构的烯基双膦氧化物。
P,C-立体异构炔丙醇RC-3 / SC-3'是通过将(L)薄荷基SPO加到丙炔中制备的,然后将其转化为P,轴向立体异构的烯基双膦氧化物。手性转移是由α-碳通过syn [2,3]-σ重排控制的。对于连接炔基部分上的弱WDG的SC-3',轴上的手性取决于立体磷。
更新日期:2020-03-25
中文翻译:

通过手性转移立体选择性地制备P,轴向立体异构的烯基双膦氧化物。
P,C-立体异构炔丙醇RC-3 / SC-3'是通过将(L)薄荷基SPO加到丙炔中制备的,然后将其转化为P,轴向立体异构的烯基双膦氧化物。手性转移是由α-碳通过syn [2,3]-σ重排控制的。对于连接炔基部分上的弱WDG的SC-3',轴上的手性取决于立体磷。


























 京公网安备 11010802027423号
京公网安备 11010802027423号