Nature ( IF 64.8 ) Pub Date : 2020-03-25 , DOI: 10.1038/s41586-020-2129-8 Huipeng Jiao 1, 2 , Laurens Wachsmuth 1, 2 , Snehlata Kumari 1, 2 , Robin Schwarzer 1, 2 , Juan Lin 1, 2 , Remzi Onur Eren 1, 2 , Amanda Fisher 3 , Rebecca Lane 3 , George R Young 4 , George Kassiotis 5, 6 , William J Kaiser 3, 7 , Manolis Pasparakis 1, 2
|
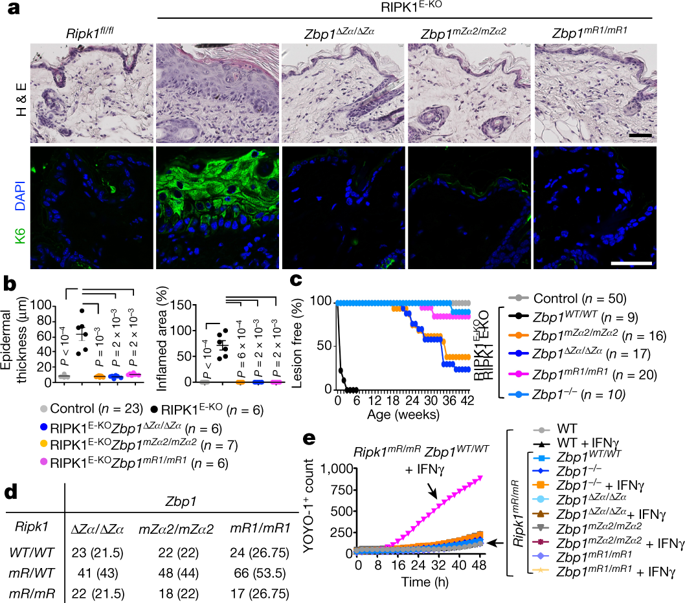
The biological function of Z-DNA and Z-RNA, nucleic acid structures with a left-handed double helix, is poorly understood1,2,3. Z-DNA-binding protein 1 (ZBP1; also known as DAI or DLM-1) is a nucleic acid sensor that contains two Zα domains that bind Z-DNA4,5 and Z-RNA6,7,8. ZBP1 mediates host defence against some viruses6,7,9,10,11,12,13,14 by sensing viral nucleic acids6,7,10. RIPK1 deficiency, or mutation of its RIP homotypic interaction motif (RHIM), triggers ZBP1-dependent necroptosis and inflammation in mice15,16. However, the mechanisms that induce ZBP1 activation in the absence of viral infection remain unknown. Here we show that Zα-dependent sensing of endogenous ligands induces ZBP1-mediated perinatal lethality in mice expressing RIPK1 with mutated RHIM (Ripk1mR/mR), skin inflammation in mice with epidermis-specific RIPK1 deficiency (RIPK1E-KO) and colitis in mice with intestinal epithelial-specific FADD deficiency (FADDIEC-KO). Consistently, functional Zα domains were required for ZBP1-induced necroptosis in fibroblasts that were treated with caspase inhibitors or express RIPK1 with mutated RHIM. Inhibition of nuclear export triggered the Zα-dependent activation of RIPK3 in the nucleus resulting in cell death, which suggests that ZBP1 may recognize nuclear Z-form nucleic acids. We found that ZBP1 constitutively bound cellular double-stranded RNA in a Zα-dependent manner. Complementary reads derived from endogenous retroelements were detected in epidermal RNA, which suggests that double-stranded RNA derived from these retroelements may act as a Zα-domain ligand that triggers the activation of ZBP1. Collectively, our results provide evidence that the sensing of endogenous Z-form nucleic acids by ZBP1 triggers RIPK3-dependent necroptosis and inflammation, which could underlie the development of chronic inflammatory conditions—particularly in individuals with mutations in RIPK1 and CASP817,18,19,20.
中文翻译:

Z-核酸感应触发 ZBP1 依赖性坏死和炎症
人们对 Z-DNA 和 Z-RNA(具有左手双螺旋结构的核酸结构)的生物学功能知之甚少1,2,3。Z-DNA 结合蛋白 1(ZBP1;也称为 DAI 或 DLM-1)是一种核酸传感器,包含两个结合 Z-DNA 4,5 和Z -RNA 6,7,8的 Zα 结构域。ZBP1通过检测病毒核酸6,7,10介导宿主防御某些病毒6,7,9,10,11,12,13,14。RIPK1 缺陷或其 RIP 同型相互作用基序 (RHIM) 的突变会触发小鼠的 ZBP1 依赖性坏死和炎症15,16. 然而,在没有病毒感染的情况下诱导 ZBP1 激活的机制仍然未知。在这里,我们表明内源性配体的 Zα 依赖性感应诱导 ZBP1 介导的围产期致死率,小鼠表达 RIPK1 与突变的 RHIM ( Ripk1 mR/mR ),表皮特异性 RIPK1 缺陷 (RIPK1 E-KO ) 小鼠的皮肤炎症和结肠炎肠上皮特异性 FADD 缺陷小鼠 (FADD IEC-KO). 一致地,ZBP1 诱导的成纤维细胞坏死性凋亡需要功能性 Zα 结构域,这些成纤维细胞用半胱天冬酶抑制剂处理或用突变的 RHIM 表达 RIPK1。核输出的抑制触发了细胞核中 RIPK3 的 Zα 依赖性激活,导致细胞死亡,这表明 ZBP1 可能识别核 Z 型核酸。我们发现 ZBP1 以 Zα 依赖性方式组成性结合细胞双链 RNA。在表皮 RNA 中检测到来自内源性逆转录元件的互补读数,这表明来自这些逆转录元件的双链 RNA 可能作为触发 ZBP1 激活的 Zα 结构域配体。总的来说,我们的结果提供了证据,证明 ZBP1 对内源性 Z 型核酸的感知触发了 RIPK3 依赖性坏死和炎症,RIPK1和CASP8 17,18,19,20。



























 京公网安备 11010802027423号
京公网安备 11010802027423号