当前位置:
X-MOL 学术
›
J. Hydrol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
The effect of groundwater velocities on sulfidation of arsenic-bearing ferrihydrite: insight from column experiments
Journal of Hydrology ( IF 6.4 ) Pub Date : 2020-07-01 , DOI: 10.1016/j.jhydrol.2020.124827 Hailong Cao , Junrong He , Xianjun Xie , Yanxin Wang , Junxia Li , Kun Qian , Yamin Deng , Yiqun Gan
Journal of Hydrology ( IF 6.4 ) Pub Date : 2020-07-01 , DOI: 10.1016/j.jhydrol.2020.124827 Hailong Cao , Junrong He , Xianjun Xie , Yanxin Wang , Junxia Li , Kun Qian , Yamin Deng , Yiqun Gan
|
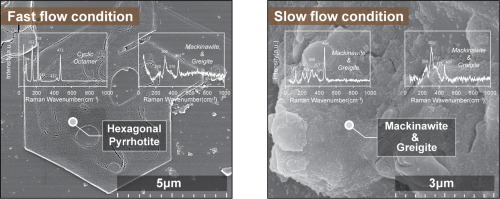
Abstract The sulfidation of Fe oxides/hydroxides has an important effect on the migration and transformation of As in groundwater. We examine the sulfidation of As (III)-bearing ferrihydrite and subsequent As repartitioning induced by sulfide in column experiments. Groundwater velocities has an obvious influence on sulfidation and the subsequent formation/transformation of secondary minerals. At a slower flow 1 pore volume·d-1), adsorbed As blocks the ferrihydrite surface, resulting in a slower sulfidation and thus slower generation of S0 and Fe2+. Mackinawite is subsequently generated by reaction of Fe2+ with excess sulfide and then reacts with S0 to form greigite. At a faster flow (5 pore volumes·d-1), stronger water elution results in desorption of much of the adsorbed As. Faster sulfidation produces considerable amounts of S0 and Fe2+ and restricts the formation of mackinawite. Fe2+ then adsorbs and catalyzes the conversion of residual ferrihydrite to hematite. The mackinawite also reacts with S0 to form pyrrhotite rather than greigite. Overall, heavy sulfidation does not effectively prevent the release of As caused by the reduction of ferrihydrite at either groundwater velocities. Sufficient As retention may be achieved by adjusting the hydrodynamic intensity of the system to determine the product of the sulfidation.
中文翻译:

地下水流速对含砷水铁矿硫化的影响:从柱实验中获得的启示
摘要 Fe氧化物/氢氧化物的硫化作用对地下水中As的迁移转化有重要影响。我们在柱实验中检查了含 As (III) 的水铁矿的硫化和随后由硫化物引起的 As 重新分配。地下水流速对硫化作用及后续次生矿物的形成/转化有明显影响。在较慢的流速 1 孔体积·d-1) 下,吸附的 As 会阻塞水铁矿表面,导致硫化较慢,从而导致 SO 和 Fe2+ 的生成较慢。随后通过 Fe2+ 与过量硫化物反应生成 Mackinawite,然后与 S0 反应形成灰泥。在较快的流速下(5 孔体积·d-1),较强的水洗脱导致大部分吸附的 As 解吸。更快的硫化会产生大量的 S0 和 Fe2+ 并限制麦金纳矿的形成。Fe2+然后吸附并催化残留的水铁矿向赤铁矿的转化。mackinawite 也与 S0 反应形成磁黄铁矿而不是绿泥石。总体而言,重硫化不能有效地阻止由水铁矿在任何地下水速度下还原引起的砷的释放。可以通过调节系统的流体力学强度来确定硫化产物,从而实现足够的砷保留。重硫化不能有效地防止水铁矿在任一地下水速度下还原引起的砷释放。可以通过调节系统的流体力学强度来确定硫化产物,从而实现足够的砷保留。重硫化不能有效地防止水铁矿在任一地下水速度下还原引起的砷释放。可以通过调节系统的流体力学强度来确定硫化产物,从而实现足够的砷保留。
更新日期:2020-07-01
中文翻译:

地下水流速对含砷水铁矿硫化的影响:从柱实验中获得的启示
摘要 Fe氧化物/氢氧化物的硫化作用对地下水中As的迁移转化有重要影响。我们在柱实验中检查了含 As (III) 的水铁矿的硫化和随后由硫化物引起的 As 重新分配。地下水流速对硫化作用及后续次生矿物的形成/转化有明显影响。在较慢的流速 1 孔体积·d-1) 下,吸附的 As 会阻塞水铁矿表面,导致硫化较慢,从而导致 SO 和 Fe2+ 的生成较慢。随后通过 Fe2+ 与过量硫化物反应生成 Mackinawite,然后与 S0 反应形成灰泥。在较快的流速下(5 孔体积·d-1),较强的水洗脱导致大部分吸附的 As 解吸。更快的硫化会产生大量的 S0 和 Fe2+ 并限制麦金纳矿的形成。Fe2+然后吸附并催化残留的水铁矿向赤铁矿的转化。mackinawite 也与 S0 反应形成磁黄铁矿而不是绿泥石。总体而言,重硫化不能有效地阻止由水铁矿在任何地下水速度下还原引起的砷的释放。可以通过调节系统的流体力学强度来确定硫化产物,从而实现足够的砷保留。重硫化不能有效地防止水铁矿在任一地下水速度下还原引起的砷释放。可以通过调节系统的流体力学强度来确定硫化产物,从而实现足够的砷保留。重硫化不能有效地防止水铁矿在任一地下水速度下还原引起的砷释放。可以通过调节系统的流体力学强度来确定硫化产物,从而实现足够的砷保留。



























 京公网安备 11010802027423号
京公网安备 11010802027423号