当前位置:
X-MOL 学术
›
Mater. Des.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Additively-manufactured poly-ether-ether-ketone (PEEK) lattice scaffolds with uniform microporous architectures for enhanced cellular response and soft tissue adhesion
Materials & Design ( IF 8.4 ) Pub Date : 2020-06-01 , DOI: 10.1016/j.matdes.2020.108671 Yanwen Su , Jiankang He , Nan Jiang , Hao Zhang , Lei Wang , Xi Liu , Dichen Li , Zhanhai Yin
Materials & Design ( IF 8.4 ) Pub Date : 2020-06-01 , DOI: 10.1016/j.matdes.2020.108671 Yanwen Su , Jiankang He , Nan Jiang , Hao Zhang , Lei Wang , Xi Liu , Dichen Li , Zhanhai Yin
|
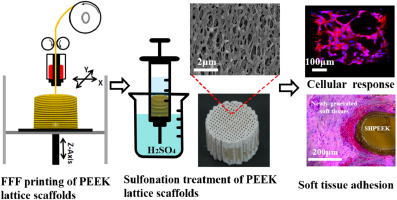
Abstract Additively-manufactured PEEK orthopedic implants have recently gained extensive attention due to their prominent characteristics such as good biocompatibility, low radiographic artifacts and similar elastic modulus to native bones. However, the inherent drawback associated with PEEK implants was their biologically inert surface which caused unsatisfactory cellular response and poor adhesion between the implants and surrounding soft tissues. Here we developed a sulfonation-treatment strategy to create microporous architectures onto the filaments of the additively-manufactured PEEK lattice scaffolds. The sulfonation time in the range of 30–45 s was found to facilitate the formation of uniform microscale pores throughout the printed PEEK lattice scaffolds and simultaneously have slight effect on their composition and mechanical properties. Biological results showed that the presence of microscale pores on the additively-manufactured PEEK lattice scaffolds significantly improved the spreading, proliferation and calcium deposition of bone-specific cells in comparison with the untreated PEEK lattice scaffolds. In vivo experiments demonstrated that the sulfonation-treated micropores facilitated the adhesion of newly-regenerated soft tissues to form tight implant-tissue bonding interfaces. The presented method provides a promising approach to improve the surface bioactivity of additively-manufactured PEEK lattice scaffolds for enhanced cellular response and soft tissue adhesion.
中文翻译:

增材制造的聚醚醚酮 (PEEK) 晶格支架具有均匀的微孔结构,可增强细胞反应和软组织粘附
摘要 增材制造的 PEEK 骨科植入物由于具有良好的生物相容性、低射线伪影和与天然骨骼相似的弹性模量等突出特点,近年来受到广泛关注。然而,与 PEEK 植入物相关的固有缺点是它们的生物惰性表面会导致不令人满意的细胞反应和植入物与周围软组织之间的粘附性差。在这里,我们开发了一种磺化处理策略,以在增材制造的 PEEK 晶格支架的细丝上创建微孔结构。发现在 30-45 s 范围内的磺化时间有助于在整个印刷的 PEEK 晶格支架上形成均匀的微孔,同时对其组成和机械性能有轻微影响。生物学结果表明,与未经处理的 PEEK 晶格支架相比,增材制造的 PEEK 晶格支架上存在的微孔显着改善了骨特异性细胞的扩散、增殖和钙沉积。体内实验表明,磺化处理的微孔促进了新再生软组织的粘附,形成了紧密的植入物-组织结合界面。所提出的方法提供了一种有前途的方法来提高增材制造的 PEEK 晶格支架的表面生物活性,以增强细胞反应和软组织粘附。与未经处理的 PEEK 晶格支架相比,骨特异性细胞的增殖和钙沉积。体内实验表明,磺化处理的微孔促进了新再生软组织的粘附,形成了紧密的植入物-组织结合界面。所提出的方法提供了一种有前途的方法来提高增材制造的 PEEK 晶格支架的表面生物活性,以增强细胞反应和软组织粘附。与未经处理的 PEEK 晶格支架相比,骨特异性细胞的增殖和钙沉积。体内实验表明,磺化处理的微孔促进了新再生软组织的粘附,形成了紧密的植入物-组织结合界面。所提出的方法提供了一种有前途的方法来提高增材制造的 PEEK 晶格支架的表面生物活性,以增强细胞反应和软组织粘附。
更新日期:2020-06-01
中文翻译:

增材制造的聚醚醚酮 (PEEK) 晶格支架具有均匀的微孔结构,可增强细胞反应和软组织粘附
摘要 增材制造的 PEEK 骨科植入物由于具有良好的生物相容性、低射线伪影和与天然骨骼相似的弹性模量等突出特点,近年来受到广泛关注。然而,与 PEEK 植入物相关的固有缺点是它们的生物惰性表面会导致不令人满意的细胞反应和植入物与周围软组织之间的粘附性差。在这里,我们开发了一种磺化处理策略,以在增材制造的 PEEK 晶格支架的细丝上创建微孔结构。发现在 30-45 s 范围内的磺化时间有助于在整个印刷的 PEEK 晶格支架上形成均匀的微孔,同时对其组成和机械性能有轻微影响。生物学结果表明,与未经处理的 PEEK 晶格支架相比,增材制造的 PEEK 晶格支架上存在的微孔显着改善了骨特异性细胞的扩散、增殖和钙沉积。体内实验表明,磺化处理的微孔促进了新再生软组织的粘附,形成了紧密的植入物-组织结合界面。所提出的方法提供了一种有前途的方法来提高增材制造的 PEEK 晶格支架的表面生物活性,以增强细胞反应和软组织粘附。与未经处理的 PEEK 晶格支架相比,骨特异性细胞的增殖和钙沉积。体内实验表明,磺化处理的微孔促进了新再生软组织的粘附,形成了紧密的植入物-组织结合界面。所提出的方法提供了一种有前途的方法来提高增材制造的 PEEK 晶格支架的表面生物活性,以增强细胞反应和软组织粘附。与未经处理的 PEEK 晶格支架相比,骨特异性细胞的增殖和钙沉积。体内实验表明,磺化处理的微孔促进了新再生软组织的粘附,形成了紧密的植入物-组织结合界面。所提出的方法提供了一种有前途的方法来提高增材制造的 PEEK 晶格支架的表面生物活性,以增强细胞反应和软组织粘附。


























 京公网安备 11010802027423号
京公网安备 11010802027423号