Journal of Inorganic Biochemistry ( IF 3.9 ) Pub Date : 2020-03-25 , DOI: 10.1016/j.jinorgbio.2020.111073 Zhijun He 1 , Menghuan Wang 2 , Qionghui Zhao 3 , Xiaoqian Li 4 , Pengan Liu 4 , Bingyu Ren 4 , Chong Wu 4 , Xiubo Du 4 , Nan Li 5 , Qiong Liu 6
|
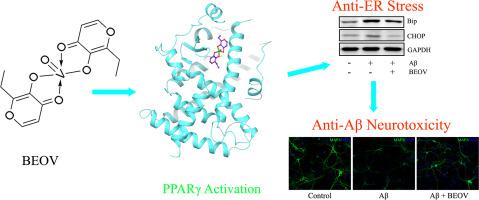
Neuronal apoptosis caused by amyloid-beta (Aβ) overproduction is one of the most important pathological features in Alzheimer's disease (AD). Endoplasmic reticulum (ER) stress induced by Aβ overload plays a critical role in this process. Bis(ethylmaltolato)oxidovanadium (IV) (BEOV), a vanadium compound which had been regarded as peroxisome proliferator-activated receptor γ (PPARγ) agonist, was reported to exert an antagonistic effect on ER stress. In this study, we tested whether BEOV could ameliorate the Aβ-induced neuronal apoptosis by inhibiting ER stress. It was observed that BEOV treatment ameliorated both tunicamycin-induced and/or Aβ-induced ER stress and neurotoxicity in a dose-dependent manner through downgrading ER stress-associated and apoptosis-associated proteins in primary hippocampal neurons. Consistent with in vitro results, BEOV also reduced ER stress and inhibited neuronal apoptosis in hippocampi and cortexes of transgenic AD model mice. Moreover, by adopting GW9662 and salubrinal, the inhibitor of PPARγ and hyperphosphorylated eukaryotic translation initiation factor 2α, respectively, we further confirmed that BEOV alleviated Aβ–induced ER stress and neuronal apoptosis in primary hippocampal neurons by activating PPARγ. Taken together, these results provided scientific evidences to support the concept that BEOV ameliorates Aβ–induced ER stress and neuronal apoptosis through activating PPARγ.
中文翻译:

双(乙基麦芽糖基)氧化钒(IV)通过激活过氧化物酶体增殖物激活的受体γ减轻了淀粉样β诱导的内质网应激引起的神经元凋亡。
淀粉样β(Aβ)过度生产引起的神经元凋亡是阿尔茨海默病(AD)最重要的病理特征之一。Aβ过载引起的内质网(ER)应力在此过程中起关键作用。据报道,被认为是过氧化物酶体增殖物激活的受体γ(PPARγ)激动剂的钒化合物双(乙基麦芽甲基)氧化钒(BEOV)对ER应激具有拮抗作用。在这项研究中,我们测试了BEOV是否可以通过抑制ER应激来改善Aβ诱导的神经元凋亡。观察到BEOV治疗通过降低初级海马神经元中与ER应激相关和凋亡相关的蛋白质,以剂量依赖性方式减轻了衣霉素诱导的和/或Aβ诱导的ER应激和神经毒性。与体外结果一致,BEOV还可以降低转基因AD模型小鼠海马和皮层的ER应激并抑制神经元凋亡。此外,通过分别采用GW9662和salubrinal作为PPARγ抑制剂和超磷酸化的真核翻译起始因子2α,我们进一步证实BEOV通过激活PPARγ减轻了Aβ诱导的原发海马神经元内质网应激和神经元凋亡。综上所述,这些结果提供了科学证据来支持BEOV通过激活PPARγ改善Aβ诱导的ER应激和神经元凋亡的概念。我们进一步证实,BEOV通过激活PPARγ减轻了Aβ诱导的ER应激和原代海马神经元的神经元凋亡。综上所述,这些结果提供了科学依据,支持BEOV通过激活PPARγ改善Aβ诱导的ER应激和神经元凋亡的概念。我们进一步证实,BEOV通过激活PPARγ减轻了Aβ诱导的ER应激和原代海马神经元的神经元凋亡。综上所述,这些结果提供了科学依据,支持BEOV通过激活PPARγ改善Aβ诱导的ER应激和神经元凋亡的概念。



























 京公网安备 11010802027423号
京公网安备 11010802027423号