当前位置:
X-MOL 学术
›
ACS Infect. Dis.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Identifying Oxacillinase-48 Carbapenemase Inhibitors Using DNA-Encoded Chemical Libraries.
ACS Infectious Diseases ( IF 5.3 ) Pub Date : 2020-03-25 , DOI: 10.1021/acsinfecdis.0c00015 Doris Mia Taylor 1 , Justin Anglin 2, 3 , Suhyeorn Park 4 , Melek N Ucisik 2, 3 , John C Faver 2, 3 , Nicholas Simmons 2, 3 , Zhuang Jin 2, 3 , Murugesan Palaniappan 2, 3 , Pranavanand Nyshadham 2, 3 , Feng Li 2, 3, 4 , James Campbell 2, 3 , Liya Hu 1 , Banumathi Sankaran 5 , B V Venkataram Prasad 1 , Hongbing Huang 2, 3, 4 , Martin M Matzuk 2, 3, 4 , Timothy Palzkill 1, 4
ACS Infectious Diseases ( IF 5.3 ) Pub Date : 2020-03-25 , DOI: 10.1021/acsinfecdis.0c00015 Doris Mia Taylor 1 , Justin Anglin 2, 3 , Suhyeorn Park 4 , Melek N Ucisik 2, 3 , John C Faver 2, 3 , Nicholas Simmons 2, 3 , Zhuang Jin 2, 3 , Murugesan Palaniappan 2, 3 , Pranavanand Nyshadham 2, 3 , Feng Li 2, 3, 4 , James Campbell 2, 3 , Liya Hu 1 , Banumathi Sankaran 5 , B V Venkataram Prasad 1 , Hongbing Huang 2, 3, 4 , Martin M Matzuk 2, 3, 4 , Timothy Palzkill 1, 4
Affiliation
|
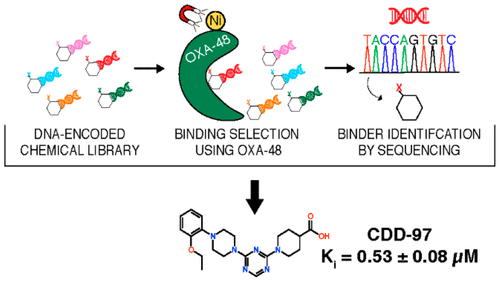
Bacterial resistance to β-lactam antibiotics is largely mediated by β-lactamases, which catalyze the hydrolysis of these drugs and continue to emerge in response to antibiotic use. β-Lactamases that hydrolyze the last resort carbapenem class of β-lactam antibiotics (carbapenemases) are a growing global health threat. Inhibitors have been developed to prevent β-lactamase-mediated hydrolysis and restore the efficacy of these antibiotics. However, there are few inhibitors available for problematic carbapenemases such as oxacillinase-48 (OXA-48). A DNA-encoded chemical library approach was used to rapidly screen for compounds that bind and potentially inhibit OXA-48. Using this approach, a hit compound, CDD-97, was identified with submicromolar potency (Ki = 0.53 ± 0.08 μM) against OXA-48. X-ray crystallography showed that CDD-97 binds noncovalently in the active site of OXA-48. Synthesis and testing of derivatives of CDD-97 revealed structure-activity relationships and informed the design of a compound with a 2-fold increase in potency. CDD-97, however, synergizes poorly with β-lactam antibiotics to inhibit the growth of bacteria expressing OXA-48 due to poor accumulation into E. coli. Despite the low in vivo activity, CDD-97 provides new insights into OXA-48 inhibition and demonstrates the potential of using DNA-encoded chemistry technology to rapidly identify β-lactamase binders and to study β-lactamase inhibition, leading to clinically useful inhibitors.
中文翻译:

使用 DNA 编码的化学文库鉴定 Oxacillinase-48 碳青霉烯酶抑制剂。
细菌对β-内酰胺类抗生素的耐药性主要由β-内酰胺酶介导,β-内酰胺酶催化这些药物的水解,并随着抗生素的使用而不断出现。水解最后的碳青霉烯类 β-内酰胺类抗生素(碳青霉烯酶)的 β-内酰胺酶是日益严重的全球健康威胁。已经开发出抑制剂来防止β-内酰胺酶介导的水解并恢复这些抗生素的功效。然而,对于有问题的碳青霉烯酶,例如苯唑西林酶-48 (OXA-48),几乎没有抑制剂可用。使用 DNA 编码的化学文库方法快速筛选结合并可能抑制 OXA-48 的化合物。使用这种方法,鉴定出针对 OXA-48 具有亚微摩尔效力 (Ki = 0.53 ± 0.08 μM) 的命中化合物 CDD-97。X 射线晶体学显示 CDD-97 与 OXA-48 的活性位点非共价结合。CDD-97 衍生物的合成和测试揭示了构效关系,并为设计一种效力提高 2 倍的化合物提供了依据。然而,CDD-97 与 β-内酰胺类抗生素的协同作用很差,无法抑制表达 OXA-48 的细菌的生长,因为它在大肠杆菌中的积累很差。尽管体内活性较低,但 CDD-97 提供了对 OXA-48 抑制的新见解,并证明了使用 DNA 编码化学技术快速识别 β-内酰胺酶结合剂和研究 β-内酰胺酶抑制的潜力,从而产生临床上有用的抑制剂。CDD-97 衍生物的合成和测试揭示了构效关系,并为设计一种效力提高 2 倍的化合物提供了依据。然而,CDD-97 与 β-内酰胺类抗生素的协同作用很差,无法抑制表达 OXA-48 的细菌的生长,因为它在大肠杆菌中的积累很差。尽管体内活性较低,但 CDD-97 提供了对 OXA-48 抑制的新见解,并证明了使用 DNA 编码化学技术快速识别 β-内酰胺酶结合剂和研究 β-内酰胺酶抑制的潜力,从而产生临床上有用的抑制剂。CDD-97 衍生物的合成和测试揭示了构效关系,并为设计一种效力提高 2 倍的化合物提供了依据。然而,CDD-97 与 β-内酰胺类抗生素的协同作用很差,无法抑制表达 OXA-48 的细菌的生长,因为它在大肠杆菌中的积累很差。尽管体内活性较低,但 CDD-97 提供了对 OXA-48 抑制的新见解,并证明了使用 DNA 编码化学技术快速识别 β-内酰胺酶结合剂和研究 β-内酰胺酶抑制的潜力,从而产生临床上有用的抑制剂。
更新日期:2020-03-17
中文翻译:

使用 DNA 编码的化学文库鉴定 Oxacillinase-48 碳青霉烯酶抑制剂。
细菌对β-内酰胺类抗生素的耐药性主要由β-内酰胺酶介导,β-内酰胺酶催化这些药物的水解,并随着抗生素的使用而不断出现。水解最后的碳青霉烯类 β-内酰胺类抗生素(碳青霉烯酶)的 β-内酰胺酶是日益严重的全球健康威胁。已经开发出抑制剂来防止β-内酰胺酶介导的水解并恢复这些抗生素的功效。然而,对于有问题的碳青霉烯酶,例如苯唑西林酶-48 (OXA-48),几乎没有抑制剂可用。使用 DNA 编码的化学文库方法快速筛选结合并可能抑制 OXA-48 的化合物。使用这种方法,鉴定出针对 OXA-48 具有亚微摩尔效力 (Ki = 0.53 ± 0.08 μM) 的命中化合物 CDD-97。X 射线晶体学显示 CDD-97 与 OXA-48 的活性位点非共价结合。CDD-97 衍生物的合成和测试揭示了构效关系,并为设计一种效力提高 2 倍的化合物提供了依据。然而,CDD-97 与 β-内酰胺类抗生素的协同作用很差,无法抑制表达 OXA-48 的细菌的生长,因为它在大肠杆菌中的积累很差。尽管体内活性较低,但 CDD-97 提供了对 OXA-48 抑制的新见解,并证明了使用 DNA 编码化学技术快速识别 β-内酰胺酶结合剂和研究 β-内酰胺酶抑制的潜力,从而产生临床上有用的抑制剂。CDD-97 衍生物的合成和测试揭示了构效关系,并为设计一种效力提高 2 倍的化合物提供了依据。然而,CDD-97 与 β-内酰胺类抗生素的协同作用很差,无法抑制表达 OXA-48 的细菌的生长,因为它在大肠杆菌中的积累很差。尽管体内活性较低,但 CDD-97 提供了对 OXA-48 抑制的新见解,并证明了使用 DNA 编码化学技术快速识别 β-内酰胺酶结合剂和研究 β-内酰胺酶抑制的潜力,从而产生临床上有用的抑制剂。CDD-97 衍生物的合成和测试揭示了构效关系,并为设计一种效力提高 2 倍的化合物提供了依据。然而,CDD-97 与 β-内酰胺类抗生素的协同作用很差,无法抑制表达 OXA-48 的细菌的生长,因为它在大肠杆菌中的积累很差。尽管体内活性较低,但 CDD-97 提供了对 OXA-48 抑制的新见解,并证明了使用 DNA 编码化学技术快速识别 β-内酰胺酶结合剂和研究 β-内酰胺酶抑制的潜力,从而产生临床上有用的抑制剂。



























 京公网安备 11010802027423号
京公网安备 11010802027423号