当前位置:
X-MOL 学术
›
J. Chem. Theory Comput.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Lennard-Jones Parameters Determined to Reproduce the Solubility of NaCl and KCl in SPC/E, TIP3P, and TIP4P/2005 Water
Journal of Chemical Theory and Computation ( IF 5.5 ) Pub Date : 2020-03-24 , DOI: 10.1021/acs.jctc.9b00941 Takuma Yagasaki 1, 2 , Masakazu Matsumoto 1, 2 , Hideki Tanaka 1, 2
Journal of Chemical Theory and Computation ( IF 5.5 ) Pub Date : 2020-03-24 , DOI: 10.1021/acs.jctc.9b00941 Takuma Yagasaki 1, 2 , Masakazu Matsumoto 1, 2 , Hideki Tanaka 1, 2
Affiliation
|
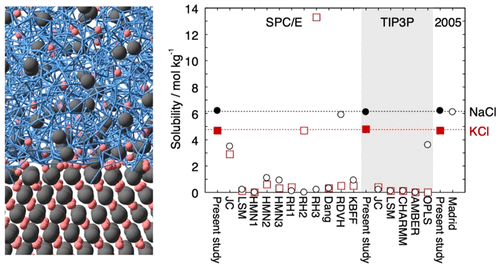
Most classical nonpolarizable ion potential models underestimate the solubility values of NaCl and KCl in water significantly. We determine Lennard-Jones parameters of Na+, K+, and Cl– that reproduce the solubility as well as the hydration free energy in dilute aqueous solutions for three water potential models, SPC/E, TIP3P, and TIP4P/2005. The ion–oxygen distance in the solution and the cation–anion distance in salt are also considered in the parametrization. In addition to the target properties, the hydration enthalpy, hydration entropy, self-diffusion coefficient, coordination number, lattice energy, enthalpy of solution, density, viscosity, and number of contact ion pairs are calculated for comparison with 17 frequently used or recently developed ion potential models. The overall performance of each ion model is represented by a global score using a scheme that was originally developed for comparison of water potential models. The global score is better for our models than for the other 17 models not only because of the quite good prediction for the solubility but also because of the relatively small deviation from the experimental value for many of the other properties.
中文翻译:

确定Lennard-Jones参数以重现NaCl和KCl在SPC / E,TIP3P和TIP4P / 2005水中的溶解度
大多数经典的非极化离子势模型都低估了NaCl和KCl在水中的溶解度值。我们确定Na +,K +和Cl –的Lennard-Jones参数可以再现三种水势模型SPC / E,TIP3P和TIP4P / 2005在稀水溶液中的溶解度以及水合自由能。在参数化中还考虑了溶液中的离子-氧气距离和盐中的阳离子-阴离子距离。除了目标特性外,还计算了水合焓,水合熵,自扩散系数,配位数,晶格能,溶液焓,密度,粘度和接触离子对数,以与17种常用或最近开发的进行比较离子电势模型。使用最初为比较水势模型而开发的方案,以总分表示每个离子模型的整体性能。
更新日期:2020-04-24
中文翻译:

确定Lennard-Jones参数以重现NaCl和KCl在SPC / E,TIP3P和TIP4P / 2005水中的溶解度
大多数经典的非极化离子势模型都低估了NaCl和KCl在水中的溶解度值。我们确定Na +,K +和Cl –的Lennard-Jones参数可以再现三种水势模型SPC / E,TIP3P和TIP4P / 2005在稀水溶液中的溶解度以及水合自由能。在参数化中还考虑了溶液中的离子-氧气距离和盐中的阳离子-阴离子距离。除了目标特性外,还计算了水合焓,水合熵,自扩散系数,配位数,晶格能,溶液焓,密度,粘度和接触离子对数,以与17种常用或最近开发的进行比较离子电势模型。使用最初为比较水势模型而开发的方案,以总分表示每个离子模型的整体性能。


























 京公网安备 11010802027423号
京公网安备 11010802027423号