当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Site-Selective and Product Chemoselective Aliphatic C–H Bond Hydroxylation of Polyhydroxylated Substrates
ACS Catalysis ( IF 12.9 ) Pub Date : 2020-03-25 , DOI: 10.1021/acscatal.9b05423 Margarida Borrell 1 , Sergio Gil-Caballero 2 , Massimo Bietti 3 , Miquel Costas 1
ACS Catalysis ( IF 12.9 ) Pub Date : 2020-03-25 , DOI: 10.1021/acscatal.9b05423 Margarida Borrell 1 , Sergio Gil-Caballero 2 , Massimo Bietti 3 , Miquel Costas 1
Affiliation
|
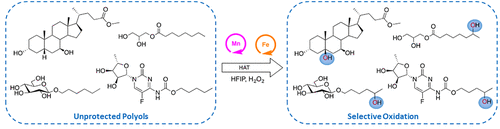
Site-selective and product chemoselective aliphatic C–H bond oxidation of 1,2-diols and of polyhydroxylated substrates using iron and manganese catalysts and hydrogen peroxide as terminal oxidant is described. The reaction capitalizes on the use of fluorinated alcohol solvents such as 2,2,2-trifluoroethanol (TFE) and 1,1,1,3,3,3-hexafluoro-2-propanol (HFIP), which exert a strong polarity reversal in the hydroxyl moieties of 1,2-diols via hydrogen bonding, in turn translating into a strong deactivation of proximal C–H bonds against a HAT initiated oxidation by the putative high-valent and electrophilic metal-oxo species. As a result, site-selective and product chemoselective oxidation of complex polyfunctional molecules such as steroids, sugars, and pharmaceuticals is described, where exclusive or predominant C–H bond hydroxylation at a remote and nonactivated site takes place. The current report discloses HAT initiated hydroxylations in fluorinated alcohol solvents as methods displaying orthogonal chemoselectivity to contemporary alcohol oxidations providing a useful tool for synthetic planning in densely functionalized molecules.
中文翻译:

多羟基化底物的位点选择性和产物化学选择性脂肪族C–H键羟基化
介绍了使用铁和锰催化剂以及过氧化氢作为末端氧化剂对1,2-二醇和多羟基化底物进行位点选择性和产物化学选择性的脂肪族CH键的氧化反应。该反应利用了氟化醇溶剂,例如2,2,2-三氟乙醇(TFE)和1,1,1,3,3,3,3-六氟-2-丙醇(HFIP),可产生强烈的极性反转1,2-二醇的羟基部分通过氢键结合,进而转化为针对HAT的近端C-H键的强烈失活,而HAT则通过假定的高价和亲电金属-氧代物质氧化。结果,描述了复杂的多官能分子(例如类固醇,糖和药物)的位点选择性氧化和产物化学选择性氧化,在偏远且未激活的位置发生独家或主要的C–H键羟化反应。本报告公开了在氟化醇溶剂中由HAT引发的羟基化反应,作为显示出对当代醇氧化具有正交化学选择性的方法,为在密集官能化分子中进行合成规划提供了有用的工具。
更新日期:2020-04-23
中文翻译:

多羟基化底物的位点选择性和产物化学选择性脂肪族C–H键羟基化
介绍了使用铁和锰催化剂以及过氧化氢作为末端氧化剂对1,2-二醇和多羟基化底物进行位点选择性和产物化学选择性的脂肪族CH键的氧化反应。该反应利用了氟化醇溶剂,例如2,2,2-三氟乙醇(TFE)和1,1,1,3,3,3,3-六氟-2-丙醇(HFIP),可产生强烈的极性反转1,2-二醇的羟基部分通过氢键结合,进而转化为针对HAT的近端C-H键的强烈失活,而HAT则通过假定的高价和亲电金属-氧代物质氧化。结果,描述了复杂的多官能分子(例如类固醇,糖和药物)的位点选择性氧化和产物化学选择性氧化,在偏远且未激活的位置发生独家或主要的C–H键羟化反应。本报告公开了在氟化醇溶剂中由HAT引发的羟基化反应,作为显示出对当代醇氧化具有正交化学选择性的方法,为在密集官能化分子中进行合成规划提供了有用的工具。



























 京公网安备 11010802027423号
京公网安备 11010802027423号