当前位置:
X-MOL 学术
›
ACS Appl. Energy Mater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Improving Electronic Conductivity of Layered Oxides through the Formation of Two-Dimensional Heterointerface for Intercalation Batteries
ACS Applied Energy Materials ( IF 6.4 ) Pub Date : 2020-03-24 00:00:00 , DOI: 10.1021/acsaem.0c00274 Mallory Clites 1 , Ryan Andris 1 , David A. Cullen 2 , Karren L. More 2 , Ekaterina Pomerantseva 1
ACS Applied Energy Materials ( IF 6.4 ) Pub Date : 2020-03-24 00:00:00 , DOI: 10.1021/acsaem.0c00274 Mallory Clites 1 , Ryan Andris 1 , David A. Cullen 2 , Karren L. More 2 , Ekaterina Pomerantseva 1
Affiliation
|
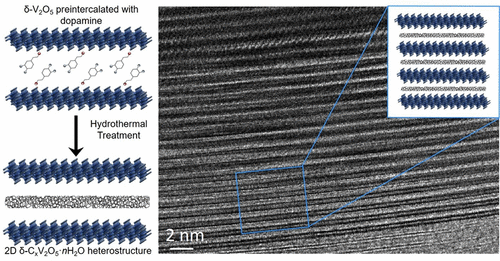
Synthetic strategies for the improvement in electronic conductivities and electrochemical stabilities of transition metal oxide cathodes, which are limiting factors in the performance of commercial intercalation batteries, are required for next-generation, high-performance battery systems. The chemical preintercalation approach, consisting of a combined sequence of a sol–gel process, extended aging, and a hydrothermal treatment, is a versatile, wet synthesis technique that allows for the incorporation of a polar species between the layers of transition metal oxides. Here, formation of a layered 2D δ-CxV2O5·nH2O heterostructure occurs via chemical preintercalation of dopamine molecules between bilayers of vanadium oxide followed by the hydrothermal treatment of the precipitate, leading to carbonization of the organic molecules. The presence of carbon layers within the structure has been confirmed via a combined analysis of scanning electron microscopy, X-ray diffraction, thermogravimetric analysis, Raman spectroscopy, X-ray photoelectron spectroscopy, electrochemical impedance spectroscopy, four-probe conductivity measurements, and scanning transmission electron microscopy characterization. 2D δ-CxV2O5·nH2O heterostructure electrodes demonstrated significantly improved electrochemical performance, particularly at higher current densities, in Li-ion cells. The heterostructure electrodes exhibited 75% of the capacity retention when the current was changed from 20 mA g–1 (206 mAh g–1) to 300 mA g–1 (155 mAh g–1), while the reference δ-V2O5·nH2O electrodes exhibited only 10% capacity retention in the same experiment. Remarkably, 2D δ-CxV2O5·nH2O heterostructure electrodes demonstrated significantly improved capacity retention (94% after 30 cycles) for bilayered vanadium oxide electrodes in Li-ion cells during galvanostatic cycling at 20 mA g–1. The improved electrochemical performance, in both extended cycling and rate capability studies, of the 2D δ-CxV2O5·nH2O heterostructure electrodes in the Li-ion system is ascribed to the intermittent formation of carbon layers within the bilayered structure, which leads to increased electronic conductivity and improved structural stability of the heterostructure compared to the reference δ-V2O5·nH2O electrodes.
中文翻译:

通过形成嵌入电池的二维异质界面提高层状氧化物的电导率
下一代高性能电池系统需要采用合成策略来改善过渡金属氧化物阴极的电子电导率和电化学稳定性,这是商用插层电池性能的限制因素。化学预插入方法由溶胶-凝胶工艺,延长的时效和水热处理的组合顺序组成,是一种通用的湿法合成技术,可在过渡金属氧化物层之间引入极性物质。在此,形成层状的2Dδ- Cx V 2 O 5 · n H 2O异质结构的发生是通过在氧化钒的双层之间化学预嵌入多巴胺分子,然后对沉淀进行水热处理,从而导致有机分子碳化。通过扫描电子显微镜,X射线衍射,热重分析,拉曼光谱,X射线光电子能谱,电化学阻抗谱,四探针电导率测量和扫描透射的综合分析已确认结构中碳层的存在电子显微镜表征。2Dδ- Cx V 2 O 5 · n H 2O异质结构电极在锂离子电池中表现出显着改善的电化学性能,特别是在较高电流密度下。所述异质结构的电极表现出的容量保持的75%时,电流从20毫安克改变-1(206毫安克-1)300 mA的克-1(155毫安克-1),而参考δ-V 2 ö在同一实验中,5 · n H 2 O电极仅表现出10%的容量保持率。显着地,2Dδ- Cx V 2 O 5 · n H 2O异质结构电极在20 mA g –1的恒电流循环过程中显示出锂离子电池中的双层钒氧化物电极的容量保持能力显着提高(30个循环后为94%)。改进的电化学性能,在这两个扩展循环和速率能力的研究中,2Dδ-C的X V 2 ø 5 · Ñ ħ 2 O异质结电极在锂离子系统是归因于内的双层间歇形成的碳层的结构,这导致增加的电子传导性和异质结构的改善的结构稳定性与参照相比δ-V 2 ø 5 · ñ ħ2个O电极。
更新日期:2020-03-24
中文翻译:

通过形成嵌入电池的二维异质界面提高层状氧化物的电导率
下一代高性能电池系统需要采用合成策略来改善过渡金属氧化物阴极的电子电导率和电化学稳定性,这是商用插层电池性能的限制因素。化学预插入方法由溶胶-凝胶工艺,延长的时效和水热处理的组合顺序组成,是一种通用的湿法合成技术,可在过渡金属氧化物层之间引入极性物质。在此,形成层状的2Dδ- Cx V 2 O 5 · n H 2O异质结构的发生是通过在氧化钒的双层之间化学预嵌入多巴胺分子,然后对沉淀进行水热处理,从而导致有机分子碳化。通过扫描电子显微镜,X射线衍射,热重分析,拉曼光谱,X射线光电子能谱,电化学阻抗谱,四探针电导率测量和扫描透射的综合分析已确认结构中碳层的存在电子显微镜表征。2Dδ- Cx V 2 O 5 · n H 2O异质结构电极在锂离子电池中表现出显着改善的电化学性能,特别是在较高电流密度下。所述异质结构的电极表现出的容量保持的75%时,电流从20毫安克改变-1(206毫安克-1)300 mA的克-1(155毫安克-1),而参考δ-V 2 ö在同一实验中,5 · n H 2 O电极仅表现出10%的容量保持率。显着地,2Dδ- Cx V 2 O 5 · n H 2O异质结构电极在20 mA g –1的恒电流循环过程中显示出锂离子电池中的双层钒氧化物电极的容量保持能力显着提高(30个循环后为94%)。改进的电化学性能,在这两个扩展循环和速率能力的研究中,2Dδ-C的X V 2 ø 5 · Ñ ħ 2 O异质结电极在锂离子系统是归因于内的双层间歇形成的碳层的结构,这导致增加的电子传导性和异质结构的改善的结构稳定性与参照相比δ-V 2 ø 5 · ñ ħ2个O电极。


























 京公网安备 11010802027423号
京公网安备 11010802027423号