当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
C–H Sulfonylation via 1,3-Rearrangement of Sulfonyl Group in N-Protected 3-Bis-sulfonimidoindole Derivatives Using Fluorine Reagent
The Journal of Organic Chemistry ( IF 3.6 ) Pub Date : 2020-03-24 , DOI: 10.1021/acs.joc.9b03498 Kazuhiro Watanabe 1 , Katsuhiko Moriyama 1
The Journal of Organic Chemistry ( IF 3.6 ) Pub Date : 2020-03-24 , DOI: 10.1021/acs.joc.9b03498 Kazuhiro Watanabe 1 , Katsuhiko Moriyama 1
Affiliation
|
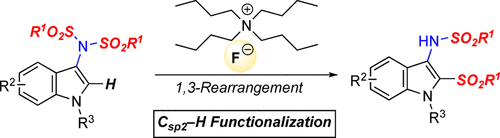
A C–H sulfonylation of N-protected 3-bis-sulfonimidoindole derivatives via a 1,3-rearrangement of a sulfonyl group on a bis-sulfonimide moiety using tetra-n-butylammonium fluoride (TBAF) was developed to provide 2-sulfonyl-3-sulfonamidoindole derivatives in high yields as a novel Csp2–H functionalization of heterocycles. The rearrangement of the sulfonyl group proceeded through an intermolecular addition of the desorbed sulfinyl ion from the bis-sulfonimide moiety in the substrate.
中文翻译:

通过含氟试剂的N保护的3-双-双磺酰亚胺氨基吲哚衍生物中磺酰基的1,3-重排使C–H磺酰化
通过使用四正丁基氟化铵(TBAF)通过双磺酰亚胺部分上的磺酰基的1,3-重排,对N保护的3-双磺酰亚胺基吲哚衍生物进行AC-H磺酰化反应,从而制得2-磺酰基-3 -磺酰胺基吲哚衍生物以高产率作为杂环的新型C sp2- H功能化。磺酰基的重排通过从底物中的双磺酰亚胺部分的解吸的亚磺酰基离子的分子间加成而进行。
更新日期:2020-04-24
中文翻译:

通过含氟试剂的N保护的3-双-双磺酰亚胺氨基吲哚衍生物中磺酰基的1,3-重排使C–H磺酰化
通过使用四正丁基氟化铵(TBAF)通过双磺酰亚胺部分上的磺酰基的1,3-重排,对N保护的3-双磺酰亚胺基吲哚衍生物进行AC-H磺酰化反应,从而制得2-磺酰基-3 -磺酰胺基吲哚衍生物以高产率作为杂环的新型C sp2- H功能化。磺酰基的重排通过从底物中的双磺酰亚胺部分的解吸的亚磺酰基离子的分子间加成而进行。



























 京公网安备 11010802027423号
京公网安备 11010802027423号