当前位置:
X-MOL 学术
›
ChemCatChem
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Chan‐Evans‐Lam C−N Coupling Promoted by a Dinuclear Positively Charged Cu(II) Complex. Catalytic Performance and Some Evidence for the Mechanism of CEL Reaction Obviating Cu(III)/Cu(I) Catalytic Cycle
ChemCatChem ( IF 4.5 ) Pub Date : 2020-03-23 , DOI: 10.1002/cctc.202000212 Nikolay Akatyev 1 , Mikhail Il'in 1 , Mikhail Il'in(Jr.) 1 , Svetlana Peregudova 1 , Alexander Peregudov 1 , Anastasiya Buyanovskaya 1 , Kirill Kudryavtsev 2 , Alexander Dubovik 1, 3 , Valerij Grinberg 1 , Victor Orlov 4 , Alexander Pavlov 1 , Valentin Novikov 1 , Ilya Volkov 1 , Yuri Belokon 1
ChemCatChem ( IF 4.5 ) Pub Date : 2020-03-23 , DOI: 10.1002/cctc.202000212 Nikolay Akatyev 1 , Mikhail Il'in 1 , Mikhail Il'in(Jr.) 1 , Svetlana Peregudova 1 , Alexander Peregudov 1 , Anastasiya Buyanovskaya 1 , Kirill Kudryavtsev 2 , Alexander Dubovik 1, 3 , Valerij Grinberg 1 , Victor Orlov 4 , Alexander Pavlov 1 , Valentin Novikov 1 , Ilya Volkov 1 , Yuri Belokon 1
Affiliation
|
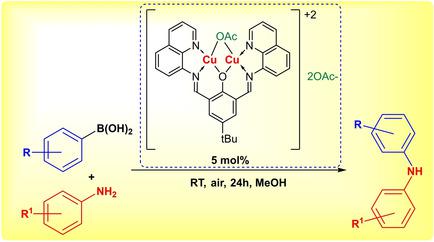
In the present study, we report the synthesis of a series of copper(II) complexes with a wide range of ligands and their testing in the copper catalyzed Chan‐Evans‐Lam (CEL) coupling of aniline and phenylboronic acid. The efficiency of the coupling was directly connected with the ease of the reduction of Cu(II) to Cu(I) of the complexes. The most efficient catalyst was derived from 4‐t ‐butyl‐2,5‐bis[(quinolinylimino)methyl]phenolate and two Cu(II) ions. Depending on the counter‐anion nature and the concentration of the reaction mixture, the reaction can be directed to predominant C−N‐bond formation. Forty‐three derivatives of diphenylamine were prepared under the optimized conditions. The proposed mechanism of the catalysis was based on the reduction potential of a series of complexes, molecular weight measurements of the catalytic complex in MeOH and the kinetic studies of aniline and phenylboronic acid coupling. In addition, an 1H NMR experiment in a sealed NMR tube, without external oxygen supply available, proved that no complete Cu(II) to Cu(I) conversion was observed under the condition, ruling out the usually accepted mechanism of the C−N coupling, which included the oxygenation of the intermediately formed Cu(I) complexes after the key step of C−N conversion had already been completed. Instead, a mechanism was proposed, involving an oxygen molecule coordinated to two copper ions in the key C−N bond formation without any detectable conversion of the Cu(II) complexes to Cu(I).
中文翻译:

由双核带正电的Cu(II)配合物促进的Chan-Evans-Lam C-N耦合。CEL反应消除Cu(III)/ Cu(I)催化循环的催化性能及一些证据
在本研究中,我们报告了一系列具有广泛配体的铜(II)配合物的合成及其在铜催化苯胺和苯基硼酸的Chan-Evans-Lam(CEL)偶联中的测试。偶联的效率与配合物的Cu(II)还原为Cu(I)的容易程度直接相关。最有效的催化剂是由4-衍生吨2,5,5-双[(喹啉基亚氨基)甲基]酚丁酸酯和两个Cu(II)离子。取决于抗衡阴离子的性质和反应混合物的浓度,反应可以直接导致CN键的形成。在最佳条件下制备了43种二苯胺衍生物。所提出的催化机理是基于一系列配合物的还原电位,在甲醇中催化配合物的分子量测量以及苯胺和苯基硼酸偶联的动力学研究。另外,1在没有外部氧气供应的情况下,在密封的NMR管中进行的H NMR实验证明,在此条件下未观察到完全的Cu(II)到Cu(I)的转化,排除了通常公认的CN偶联机理,包括在完成CN转换的关键步骤后,中间形成的Cu(I)络合物的氧合作用已经完成。取而代之的是,提出了一种机制,该机制涉及在关键的C-N键形成过程中与两个铜离子配位的氧分子,而没有任何可检测到的Cu(II)配合物向Cu(I)的转化。
更新日期:2020-03-23
中文翻译:

由双核带正电的Cu(II)配合物促进的Chan-Evans-Lam C-N耦合。CEL反应消除Cu(III)/ Cu(I)催化循环的催化性能及一些证据
在本研究中,我们报告了一系列具有广泛配体的铜(II)配合物的合成及其在铜催化苯胺和苯基硼酸的Chan-Evans-Lam(CEL)偶联中的测试。偶联的效率与配合物的Cu(II)还原为Cu(I)的容易程度直接相关。最有效的催化剂是由4-衍生吨2,5,5-双[(喹啉基亚氨基)甲基]酚丁酸酯和两个Cu(II)离子。取决于抗衡阴离子的性质和反应混合物的浓度,反应可以直接导致CN键的形成。在最佳条件下制备了43种二苯胺衍生物。所提出的催化机理是基于一系列配合物的还原电位,在甲醇中催化配合物的分子量测量以及苯胺和苯基硼酸偶联的动力学研究。另外,1在没有外部氧气供应的情况下,在密封的NMR管中进行的H NMR实验证明,在此条件下未观察到完全的Cu(II)到Cu(I)的转化,排除了通常公认的CN偶联机理,包括在完成CN转换的关键步骤后,中间形成的Cu(I)络合物的氧合作用已经完成。取而代之的是,提出了一种机制,该机制涉及在关键的C-N键形成过程中与两个铜离子配位的氧分子,而没有任何可检测到的Cu(II)配合物向Cu(I)的转化。


























 京公网安备 11010802027423号
京公网安备 11010802027423号