当前位置:
X-MOL 学术
›
ChemCatChem
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Rigidifying Ag(I) Complexes for Selective Nitrene Transfer
ChemCatChem ( IF 4.5 ) Pub Date : 2020-03-23 , DOI: 10.1002/cctc.202000336 Minxue Huang 1 , Jon Paretsky 1 , Jennifer M. Schomaker 1
ChemCatChem ( IF 4.5 ) Pub Date : 2020-03-23 , DOI: 10.1002/cctc.202000336 Minxue Huang 1 , Jon Paretsky 1 , Jennifer M. Schomaker 1
Affiliation
|
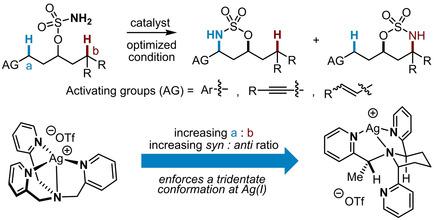
One of the challenges in achieving tunable and predictable C−H bond amidation via silver‐catalyzed nitrene transfer (NT) is the conformational flexibility and dynamic nature of many Ag(I) complexes. This fluxional behavior can make it difficult to obtain a clear understanding of the factors responsible for influencing the site‐selectivity of the amidation event. Mechanistic studies on related systems suggest that π ⋅⋅⋅π and Ag⋅⋅⋅π interactions play important roles in altering the preference for nitrene transfer at sites adjacent to π systems, including benzylic, allylic, and propargylic C−H bonds. In this paper, we report a Ag(I) catalyst designed to improve the preference for reaction at benzylic, allylic, and propargylic C−H bonds over more electron‐rich tertiary alkyl C(sp3)−H bonds by enhancing non‐covalent interactions. Rigidifying a flexible tris(2‐pyridylmethyl)amine ligand scaffold by tying back the sp3 nitrogen into a piperidine ring strengthened non‐covalent interactions between the π‐containing substituents on the substrate and the pyridine arm of the catalyst and improved the site‐selectivity of the nitrene transfer event.
中文翻译:

刚性的Ag(I)配合物用于选择性的硝基转移
通过银催化的氮烯转移(NT)实现可调和可预测的CH键酰胺化的挑战之一是许多Ag(I)配合物的构象柔韧性和动力学性质。这种流动行为可能使人们很难清楚地了解影响酰胺化反应位点选择性的因素。在相关制度机理研究表明,π ⋅⋅⋅ π和Ag⋅⋅⋅ π相互作用在改变相邻于π系统(包括苄基,烯丙基和炔丙基CH键)的位点上转移丁腈的偏好方面起着重要作用。在本文中,我们报告了一种Ag(I)催化剂,旨在通过增强非共价键来提高对电子富集的叔烷基C(sp 3)-H键的反应,从而提高对苄基,烯丙基和炔丙基CH键的反应偏好。互动。通过将sp 3氮束缚到哌啶环中来刚性化柔性三(2-吡啶基甲基)胺配体支架,从而增强了底物上含π的取代基与催化剂的吡啶臂之间的非共价相互作用,并提高了位点选择性的氮转移事件。
更新日期:2020-03-23
中文翻译:

刚性的Ag(I)配合物用于选择性的硝基转移
通过银催化的氮烯转移(NT)实现可调和可预测的CH键酰胺化的挑战之一是许多Ag(I)配合物的构象柔韧性和动力学性质。这种流动行为可能使人们很难清楚地了解影响酰胺化反应位点选择性的因素。在相关制度机理研究表明,π ⋅⋅⋅ π和Ag⋅⋅⋅ π相互作用在改变相邻于π系统(包括苄基,烯丙基和炔丙基CH键)的位点上转移丁腈的偏好方面起着重要作用。在本文中,我们报告了一种Ag(I)催化剂,旨在通过增强非共价键来提高对电子富集的叔烷基C(sp 3)-H键的反应,从而提高对苄基,烯丙基和炔丙基CH键的反应偏好。互动。通过将sp 3氮束缚到哌啶环中来刚性化柔性三(2-吡啶基甲基)胺配体支架,从而增强了底物上含π的取代基与催化剂的吡啶臂之间的非共价相互作用,并提高了位点选择性的氮转移事件。



























 京公网安备 11010802027423号
京公网安备 11010802027423号