当前位置:
X-MOL 学术
›
ChemistrySelect
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Recyclization‐Isomerization in the Reduction of 1‐(2‐Nitro(het)aryl)benzimidazoles
ChemistrySelect ( IF 2.1 ) Pub Date : 2020-03-24 , DOI: 10.1002/slct.201904898 Roman S. Begunov 1 , Artyom N. Fakhrutdinov 2 , Aleksandr A. Sokolov 1
ChemistrySelect ( IF 2.1 ) Pub Date : 2020-03-24 , DOI: 10.1002/slct.201904898 Roman S. Begunov 1 , Artyom N. Fakhrutdinov 2 , Aleksandr A. Sokolov 1
Affiliation
|
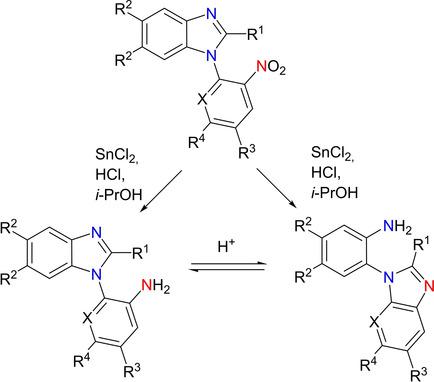
The regularities of reductive recyclization‐isomerization of 1‐(2‐nitro(het)aryl)‐substituted benzimidazoles discovered by the authors have been studied. The structure of the intermediate has been identified and the isomerization process has been found to be reversible. The shift of equilibrium between the resulting isomers is affected by the reaction temperature and time, the amount of hydrogen chloride in the reaction mixture and the substrate structure. According to the suggested reaction mechanism, the amino group formed upon reduction reacts with the electron‐deficient α‐carbon atom in an imidazole by an AdN reaction. Based on these studies, a method for the functionalization of benzimidazoles has been developed. This young methodology has been used to synthesize hitherto unknown benzimidazole derivatives that are difficult to obtain by other methods.
中文翻译:

还原1-(2-硝基(杂)芳基)苯并咪唑类化合物中的环化异构化
作者研究了1-(2-硝基(杂)芳基)取代的苯并咪唑的还原性循环异构化的规律。已经确定了中间体的结构,并且发现异构化过程是可逆的。所得异构体之间的平衡位移受反应温度和时间,反应混合物中氯化氢的量以及底物结构的影响。根据建议的反应机理,还原后形成的氨基通过Ad N与咪唑中缺电子的α-碳原子反应反应。基于这些研究,已经开发了用于苯并咪唑官能化的方法。这种年轻的方法已被用于合成迄今未知的苯并咪唑衍生物,而其他方法难以获得。
更新日期:2020-03-24
中文翻译:

还原1-(2-硝基(杂)芳基)苯并咪唑类化合物中的环化异构化
作者研究了1-(2-硝基(杂)芳基)取代的苯并咪唑的还原性循环异构化的规律。已经确定了中间体的结构,并且发现异构化过程是可逆的。所得异构体之间的平衡位移受反应温度和时间,反应混合物中氯化氢的量以及底物结构的影响。根据建议的反应机理,还原后形成的氨基通过Ad N与咪唑中缺电子的α-碳原子反应反应。基于这些研究,已经开发了用于苯并咪唑官能化的方法。这种年轻的方法已被用于合成迄今未知的苯并咪唑衍生物,而其他方法难以获得。


























 京公网安备 11010802027423号
京公网安备 11010802027423号