当前位置:
X-MOL 学术
›
Adv. Synth. Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
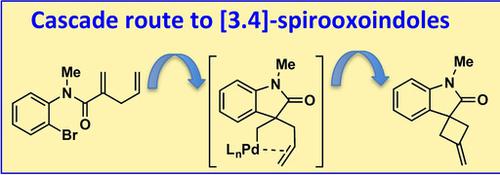
Synthesis of [3.4]‐Spirooxindoles through Cascade Carbopalladation of Skipped Dienes
Advanced Synthesis & Catalysis ( IF 5.4 ) Pub Date : 2020-04-06 , DOI: 10.1002/adsc.202000111 Hamid Azizollahi 1, 2 , Marta Pérez‐Gómez 1 , Vaibhav P. Mehta 1 , José‐Antonio García‐López 1
Advanced Synthesis & Catalysis ( IF 5.4 ) Pub Date : 2020-04-06 , DOI: 10.1002/adsc.202000111 Hamid Azizollahi 1, 2 , Marta Pérez‐Gómez 1 , Vaibhav P. Mehta 1 , José‐Antonio García‐López 1
Affiliation
|
A synthetic route to [3.4]‐spirooxindoles based on cascade carbopalladation reactions of 1,4‐dienes is described. While carbopalladation of alkenes have been used to access mainly [4.4]‐ or [4.5]‐spirocycles, 4‐exo‐trig carbopalladation has not been yet applied to the synthesis of relevant [3.4]‐spirooxindole scaffolds bearing a cyclobutyl ring. In addition, the cascade reaction generates an exocyclic double bond that can serve as a platform to further diversify the substitution pattern of the spirooxindole nuclei.
中文翻译:

跳过的二烯的级联碳钯反应合成[3.4]-螺氧杂吲哚
描述了基于1,4-二烯级联碳car反应的合成[3.4]-螺毒素的合成途径。虽然烯烃的碳链共轭主要用于获得[4.4]-或[4.5]-螺环,但4- exo - trig碳链共轭尚未用于合成带有环丁基环的[3.4]-螺氧并恶唑骨架。另外,级联反应产生环外双键,该环外双键可以用作进一步使螺氧并吲哚核的取代方式多样化的平台。
更新日期:2020-04-06
中文翻译:

跳过的二烯的级联碳钯反应合成[3.4]-螺氧杂吲哚
描述了基于1,4-二烯级联碳car反应的合成[3.4]-螺毒素的合成途径。虽然烯烃的碳链共轭主要用于获得[4.4]-或[4.5]-螺环,但4- exo - trig碳链共轭尚未用于合成带有环丁基环的[3.4]-螺氧并恶唑骨架。另外,级联反应产生环外双键,该环外双键可以用作进一步使螺氧并吲哚核的取代方式多样化的平台。



























 京公网安备 11010802027423号
京公网安备 11010802027423号